Evaluation of three rapid assays for detecting Mycoplasma pneumoniae in nasopharyngeal specimens
- PMID: 39694968
- PMCID: PMC11655899
- DOI: 10.1186/s13568-024-01782-5
Evaluation of three rapid assays for detecting Mycoplasma pneumoniae in nasopharyngeal specimens
Abstract
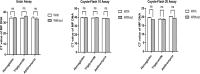
During the 2023 autumn-winter period in China, Mycoplasma pneumoniae (MP) infections have increased. To address this, rapid and accurate MP DNA detection methods are crucial. Three nucleic acid detection assays (Ustar, Coyote Flash10, Coyote Flash 20) that are widely used in China are currently being evaluated for their effectiveness in detecting MP DNA in nasopharyngeal specimens. Reference standard materials for MP and a total of 35 NPS collected from Peking Union Medical College Hospital were tested using the Ustar, Coyote Flash10 and Coyote Flash 20 assays to assess analytical sensitivity, analytical specificity, diagnostic performance and workflow. The assays showed differing limits of detection (LOD) based on the absolute quantification of reference standards, with LODs of 500 copies/mL for the Ustar assays and 200 copies/mL for both Coyote Flash10 and Coyote Flash 20 assays. Additionally, all three assays displayed excellently analytical specificity in detecting MP DNA.The clinical correlation analysis demonstrated that the Ustar assay exhibited a sensitivity of 90.00%, specificity of 100%, positive predictive value (PPV) of 100%, and negative predictive value (NPV) of 62.50%. In contrast, both the Coyote Flash10 and Coyote Flash 20 assays displayed perfect diagnostic accuracy with 100% sensitivities, specificities, PPVs, and NPVs. Despite variations in detection principles, sample volume, and pre-preparation among the three assays, they all had a turnaround time of less than 30 min with low-throughput processing. Overall, all three rapid nucleic acid detection assays displayed excellent clinical performance in detecting MP DNA, offering a solid foundation for the quick clinical diagnosis of MP infection.
Keywords: Mycoplasma pneumoniae; Analytical sensitivity; Analytical specificity; Diagnostic performance, workflow; Rapid nucleic acid detection assays.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee at PUMCH. Competing interests: The authors declare that they have no competing interests.
Figures
Similar articles
-
Analytical Performance Evaluation of Three Commercial Rapid Nucleic Acid Assays for SARS-CoV-2.Infect Drug Resist. 2021 Aug 14;14:3169-3174. doi: 10.2147/IDR.S321227. eCollection 2021. Infect Drug Resist. 2021. PMID: 34429616 Free PMC article.
-
[Analytical performance evaluation of Flash10 point-of-care testing in severe acute respiratory syndrome coronavirus 2 nucleic acid detection].Zhonghua Yi Xue Za Zhi. 2024 Dec 17;104(47):4323-4329. doi: 10.3760/cma.j.cn112137-20240719-01661. Zhonghua Yi Xue Za Zhi. 2024. PMID: 39667770 Chinese.
-
Analytical performance of rapid nucleic acid detection assays and routine RT-qPCR assays for detection of SARS-CoV-2 in Shanghai, China in 2022.Diagn Microbiol Infect Dis. 2023 Feb;105(2):115860. doi: 10.1016/j.diagmicrobio.2022.115860. Epub 2022 Nov 12. Diagn Microbiol Infect Dis. 2023. PMID: 36459887 Free PMC article.
-
Comparison of sputum and nasopharyngeal swab specimens for molecular diagnosis of Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila.Ann Lab Med. 2012 Mar;32(2):133-8. doi: 10.3343/alm.2012.32.2.133. Epub 2012 Feb 23. Ann Lab Med. 2012. PMID: 22389880 Free PMC article.
-
Immunochromatography for the diagnosis of Mycoplasma pneumoniae infection: A systematic review and meta-analysis.PLoS One. 2020 Mar 17;15(3):e0230338. doi: 10.1371/journal.pone.0230338. eCollection 2020. PLoS One. 2020. PMID: 32182283 Free PMC article.
Cited by
-
Implementation of a Laboratory-Developed Test for the Diagnosis of Mycoplasma pneumoniae Using a High-Throughput Approach.Pathogens. 2025 Jul 14;14(7):692. doi: 10.3390/pathogens14070692. Pathogens. 2025. PMID: 40732737 Free PMC article.
References
-
- Abdulhadi B, Kiel J (2023) StatPearls. Treasure Island (FL)
-
- Amaral C, Antunes W, Moe E, Duarte AG, Lima L, Santos C, Gomes IL, Afonso GS, Vieira R, Teles H, Reis MS, da Silva M, Henriques AM, Fevereiro M, Ventura MR, Serrano M, Pimentel C (2021) A molecular test based on RT-LAMP for rapid, sensitive and inexpensive colorimetric detection of SARS-CoV-2 in clinical samples. Sci Rep 11:16430. 10.1038/s41598-021-95799-6 - PMC - PubMed
-
- Cao G, Lin K, Ai J, Cai J, Zhang H, Yu Y, Liu Q, Zhang X, Zhang Y, Fu Z, Song J, Wang H, Yuan G, Wang S, Guan M, Zhang W (2022) A diagnostic accuracy study comparing RNA LAMP, direct LAMP, and rapid antigen testing from nasopharyngeal swabs. Front Microbiol 13:1063414. 10.3389/fmicb.2022.1063414 - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources