Elranatamab monotherapy in the real-word setting in relapsed-refractory multiple myeloma: results of the French compassionate use program on behalf of the IFM
- PMID: 39695076
- PMCID: PMC11655550
- DOI: 10.1038/s41408-024-01200-w
Elranatamab monotherapy in the real-word setting in relapsed-refractory multiple myeloma: results of the French compassionate use program on behalf of the IFM
Conflict of interest statement
Competing interests: F.M. reports honoraria from Therakos/Mallinckrodt, Janssen, Biocodex, Sanofi, JAZZ Pharmaceuticals, Gilead, Novartis, Priothera, and Astellas, all outside the submitted work. L.K. reports honoraria, advisory board participation and travel expenses from Pfizer, Janssen, Takeda, Sanofi and BMS, all outside the submitted work. L.F. reports honoraria from Janssen, Sanofi, Pfizer and Abbvie all outside the submitted work. E.C. reports honoraria for lectures from Sanofi, Janssen, Amgen, Stemline, and for advisory board participation from BMS, Sanofi, Stemline, Pfizer and Janssen, all outside the submitted work. C.C. reports research grants from Abbvie, and Janssen; consulting fees, honoraria, and/or travel funds from Abbvie, Janssen, Sanofi, AstraZeneca, and Novartis, all outside the submitted work. X.L. reports honoraria and consultancy from Janssen. Pfizer. Gilead kite, Novartis, Roche, Takeda, Sanofi, Astra Zeneca, BMS, GSK, Abbvie, and Amgen, all outside the submitted work. A.P. reports honoraria from Abbvie, Amgen, Janssen, GSK, BMS, Sanofi, Pfizer and Takeda, all outside the submitted work. M.M. reports grants and honoraria from Janssen, Sanofi, and JAZZ Pharmaceuticals, honoraria from Celgene, Amgen, MaaT Pharma, BMS, Takeda, and Pfizer, and grants from Roche, all outside the submitted work. The other authors did not disclose any relevant conflict of interest.
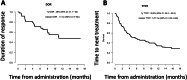
Figures
References
Publication types
LinkOut - more resources
Full Text Sources