The clinically relevant MEK inhibitor mirdametinib combined with D-cycloserine and prediction error disrupts fear memory in PTSD models
- PMID: 39695081
- PMCID: PMC11655561
- DOI: 10.1038/s41398-024-03190-6
The clinically relevant MEK inhibitor mirdametinib combined with D-cycloserine and prediction error disrupts fear memory in PTSD models
Abstract
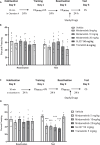
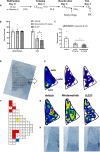
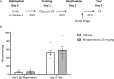
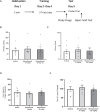
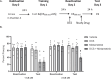
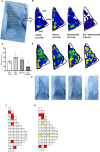
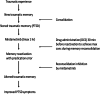
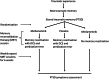
This study establishes mirdametinib as the first MEK inhibitor that can undergo clinical development for psychiatric indications such as post-traumatic stress disorder (PTSD). PTSD is characterized by persistent traumatic memories with limited effective treatment options. A body of evidence suggests that memory storage is dynamic and constantly updated through post-retrieval modification a process termed reconsolidation. Although ERK/MAPK signaling plays a central role in fear memory consolidation, no clinically translatable MEK inhibitor has been tested in experimental models or in clinical trials to disrupt this process. Furthermore, there is need to develop pharmacological and behavioral strategies to labilize the memory to make it susceptible for disruption. Here, we disrupted fear memory reconsolidation with the clinically relevant MEK inhibitor mirdametinib in C57BL/6 mice and tested memory destabilization strategies using an auditory fear conditioning paradigm, with drugs administered following reactivation of memory. We found prediction error effective in labilizing weak fear memory and combined D-cycloserine (DCS) and predication error effective in labilizing strong fear memory. Mirdametinib disrupted the weak fear memory and reduced ERK phosphorylation in lateral amygdala when coupled with prediction error at the time of memory reactivation but required coordinated combination of DCS, prediction error and mirdametinib to disrupt strong fear memory. Barnes maze spatial memory test and open field test revealed that mirdametinib did not affect retrieval of other forms (spatial) of long-term memory and locomotor activity. Furthermore, the effect of mirdametinib was specific to reconsolidation as it had no effect on fear memory when given without reactivation. These translational findings identify a new drug that can be adapted for the treatment of PTSD.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Breslau N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? J Clin Psychiatry. 2001;62:16–22. - PubMed
-
- Wynn GH, Benedek DM, Johnson L, Choi K, Zhang L, Hu XZ, et al. Chapter 22—Posttraumatic stress disorder. In: Conn PM, editor. Conn’s translational neuroscience. San Diego: Academic Press; 2017. pp. 499–515.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

