GPx1 deficiency confers increased susceptibility to ferroptosis in macrophages from individuals with active Crohn's disease
- PMID: 39695083
- PMCID: PMC11655851
- DOI: 10.1038/s41419-024-07289-y
GPx1 deficiency confers increased susceptibility to ferroptosis in macrophages from individuals with active Crohn's disease
Abstract
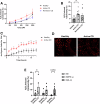
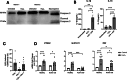
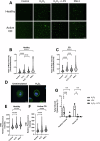
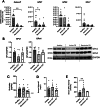
Intestinal cell death is a defining feature of Crohn's disease (CD), a major form of inflammatory bowel disease. The focus on this aspect of enteric inflammation has mainly been on epithelial cells, while other cell types such as stromal and myeloid cells have received less attention. Hypothesising that decreased macrophage viability in an oxidative environment could be a contributing factor to the pathophysiology of CD, we found that monocyte-derived macrophages from individuals with active CD (but not those in clinical disease remission) have increased sensitivity to cell death induced by H2O2. Molecular biology and pharmacological studies ruled out apoptosis and necroptosis, while increased lipid peroxidation and surface expression of the transferrin receptor implicated ferroptosis as the mechanism of the H2O2-induced cell death: this was supported by suppression of H2O2-cytotoxicity by liproxstatin-1, a pharmacological inhibitor of ferroptosis. Selenoproteins are important antioxidants, and selenium deficiency can be a feature of CD. Despite normal dietary intake of selenium, monocyte-derived macrophages and intestinal macrophages in individuals with CD had decreased protein and/or mRNA expression of the selenoprotein, glutathione peroxidase (GPx)-1. Knockdown of GPx1 in macrophages from healthy volunteers resulted in increased H2O2-induced cell death reminiscent of that observed with macrophages from CD. In summary, monocyte-derived macrophages from individuals with CD have increased susceptibility to H2O2-induced ferroptosis cell death, that may be facilitated, at least in part, by reduced expression of the antioxidant GPx1. We suggest that reduced GPx1 in monocytes recruited to the gut and intestinal macrophages renders these cells vulnerable to reactive oxygen species-evoked ferroptosis cell death and that unraveling the participation of this pathway in Crohn's disease may reveal novel therapeutic approaches to this chronic condition.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: Ethics approval was issued by the Conjoint Health Research Ethics Board at the University of Calgary (REB24827, REB15-1270, REB19-0402, and REB 20-1534) and the study was performed under accordance with the Declaration of Helsinki.
Figures


References
-
- Moreira Lopes TC, Mosser DM, Goncalves R. Macrophage polarization in intestinal inflammation and gut homeostasis. Inflamm Res. 2020;69:1163–72. - PubMed
-
- Hunter MM, Wang A, Parhar KS, Johnston MJ, Van Rooijen N, Beck PL, et al. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–405. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

