Metabolic shifts in lipid utilization and reciprocal interactions within the lung metastatic niche of triple-negative breast cancer revealed by spatial multi-omics
- PMID: 39695088
- PMCID: PMC11655832
- DOI: 10.1038/s41419-024-07205-4
Metabolic shifts in lipid utilization and reciprocal interactions within the lung metastatic niche of triple-negative breast cancer revealed by spatial multi-omics
Abstract
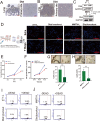
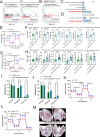
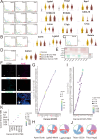
The Triple-Negative Breast Cancer (TNBC) subtype constitutes 15-20% of breast cancer cases and is associated with the poorest clinical outcomes. Distant metastasis, particularly to the lungs, is a major contributor to the high mortality rates in breast cancer patients. Despite this, there has been a lack of comprehensive insights into the heterogeneity of metastatic tumors and their surrounding ecosystem in the lungs. In this study, we utilized spatial RNA sequencing technology to investigate the heterogeneity of lung metastatic tumors and their microenvironment in two spontaneous lung metastatic mouse models. Our findings revealed an increase in metabolic-related genes within the cancer cells, with the hub gene Dlat (Dihydrolipoamide S-Acetyltransferase) showing a significant association with the development of lung metastatic tumors. Upregulation of Dlat led to the reprogramming of fatty acid utilization, markedly enhancing the bioenergetic capacity of cancer cells. This finding was corroborated by the increased dependence on fatty acid utilization in lung metastatic cancer cells, and inhibition of Dlat in breast cancer cells exhibited a reduced oxygen consumption rate. Consequently, inhibition of Dlat resulted in decreased survival capacity of breast cancer by reducing cancer stem cell properties and cell adhesion in the lung in vivo. The three cell components within the lung metastatic niche, including CD163+ macrophages, neutrophils, and endothelial cells, expressed elevated levels of ApoE, leading to the secretion of various protumorigenic molecules that promote cancer cell growth in the lung. These molecules include galectin-1, S100A10, S100A4, and S100A6. Collectively, our findings highlight the lipid metabolism reprogramming of cancer and components of the tumor microenvironment that support lung metastasis of TNBC breast cancer.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: Ethics approval for animals in this project was obtained from the Institutional Animal. Care and Use Committee (IACUC) in Kaohsiung Medical University (approval no. 112118). All methods were performed in accordance with relevant guidelines and regulations.
Figures




References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17–48. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

