Role of N6-methyladenosine methylation in nasopharyngeal carcinoma: current insights and future prospective
- PMID: 39695216
- PMCID: PMC11655975
- DOI: 10.1038/s41420-024-02266-y
Role of N6-methyladenosine methylation in nasopharyngeal carcinoma: current insights and future prospective
Abstract
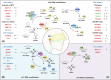
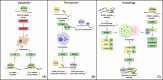
Nasopharyngeal carcinoma (NPC) is a distinct type of head and neck squamous cell carcinoma prevalent in Southern China, Southeast Asia, and North Africa. Despite advances in treatment options, the prognosis for advanced NPC remains poor, underscoring the urgent need to explore its underlying mechanisms and develop novel therapeutic strategies. Epigenetic alterations have been shown to play a key role in NPC progression. Recent studies indicate that dysregulation of RNA modifications in NPC specifically affects tumor-related transcripts, influencing various oncogenic processes. This review provides a comprehensive overview of altered RNA modifications and their regulators in NPC, with a focus on m6A and its regulatory mechanisms. We discuss how m6A RNA modification influences gene expression and affects NPC initiation and progression at the molecular level, analyzing its impact on cancer-related biological functions. Understanding these modifications could reveal new biomarkers and therapeutic targets for NPC, offering promising directions for future research and precision medicine.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: Not applicable.
Figures



References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. 10.3322/caac.21492 - PubMed
-
- Wong KCW, Hui EP, Lo KW, Lam WKJ, Johnson D, Li L, et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol. 2021;18:679–95. 10.1038/s41571-021-00524-x - PubMed
-
- Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. 10.1016/s0140-6736(19)30956-0 - PubMed
-
- Carioli G, Negri E, Kawakita D, Garavello W, La Vecchia C, Malvezzi M. Global trends in nasopharyngeal cancer mortality since 1970 and predictions for 2020: Focus on low-risk areas. Int J Cancer. 2017;140:2256–64. 10.1002/ijc.30660 - PubMed
-
- Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16:645–55. 10.1016/s1470-2045(15)70126-9 - PubMed
Publication types
Grants and funding
- 82203847/National Natural Science Foundation of China (National Science Foundation of China)
- 82203430/National Natural Science Foundation of China (National Science Foundation of China)
- SL2024A04J00877, 202201010139/Guangzhou Science and Technology Program key projects
- A2022107/Guangdong Medical Research Foundation (Guangdong Province Medical Research Foundation)
- 2022A1515110078/Guangdong Medical Research Foundation (Guangdong Province Medical Research Foundation)
LinkOut - more resources
Full Text Sources

