Lithocholic acid phenocopies anti-ageing effects of calorie restriction
- PMID: 39695227
- PMCID: PMC12222012
- DOI: 10.1038/s41586-024-08329-5
Lithocholic acid phenocopies anti-ageing effects of calorie restriction
Erratum in
-
Author Correction: Lithocholic acid phenocopies anti-ageing effects of calorie restriction.Nature. 2025 Feb;638(8050):E6. doi: 10.1038/s41586-025-08693-w. Nature. 2025. PMID: 39875609 Free PMC article. No abstract available.
Abstract
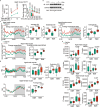
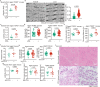
Calorie restriction (CR) is a dietary intervention used to promote health and longevity1,2. CR causes various metabolic changes in both the production and the circulation of metabolites1; however, it remains unclear which altered metabolites account for the physiological benefits of CR. Here we use metabolomics to analyse metabolites that exhibit changes in abundance during CR and perform subsequent functional validation. We show that lithocholic acid (LCA) is one of the metabolites that alone can recapitulate the effects of CR in mice. These effects include activation of AMP-activated protein kinase (AMPK), enhancement of muscle regeneration and rejuvenation of grip strength and running capacity. LCA also activates AMPK and induces life-extending and health-extending effects in Caenorhabditis elegans and Drosophila melanogaster. As C. elegans and D. melanogaster are not able to synthesize LCA, these results indicate that these animals are able to transmit the signalling effects of LCA once administered. Knockout of AMPK abrogates LCA-induced phenotypes in all the three animal models. Together, we identify that administration of the CR-mediated upregulated metabolite LCA alone can confer anti-ageing benefits to metazoans in an AMPK-dependent manner.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures












References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

