Hierarchical design of pseudosymmetric protein nanocages
- PMID: 39695230
- PMCID: PMC11821544
- DOI: 10.1038/s41586-024-08360-6
Hierarchical design of pseudosymmetric protein nanocages
Abstract
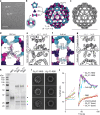
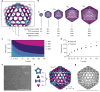
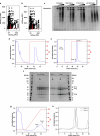
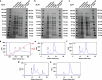
Discrete protein assemblies ranging from hundreds of kilodaltons to hundreds of megadaltons in size are a ubiquitous feature of biological systems and perform highly specialized functions1,2. Despite remarkable recent progress in accurately designing new self-assembling proteins, the size and complexity of these assemblies has been limited by a reliance on strict symmetry3. Here, inspired by the pseudosymmetry observed in bacterial microcompartments and viral capsids, we developed a hierarchical computational method for designing large pseudosymmetric self-assembling protein nanomaterials. We computationally designed pseudosymmetric heterooligomeric components and used them to create discrete, cage-like protein assemblies with icosahedral symmetry containing 240, 540 and 960 subunits. At 49, 71 and 96 nm diameter, these nanocages are the largest bounded computationally designed protein assemblies generated to date. More broadly, by moving beyond strict symmetry, our work substantially broadens the variety of self-assembling protein architectures that are accessible through design.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






Update of
-
Hierarchical design of pseudosymmetric protein nanoparticles.bioRxiv [Preprint]. 2023 Jun 17:2023.06.16.545393. doi: 10.1101/2023.06.16.545393. bioRxiv. 2023. Update in: Nature. 2025 Feb;638(8050):553-561. doi: 10.1038/s41586-024-08360-6. PMID: 37398374 Free PMC article. Updated. Preprint.
-
Hierarchical design of pseudosymmetric protein nanoparticles.Res Sq [Preprint]. 2023 Jul 10:rs.3.rs-3074553. doi: 10.21203/rs.3.rs-3074553/v1. Res Sq. 2023. Update in: Nature. 2025 Feb;638(8050):553-561. doi: 10.1038/s41586-024-08360-6. PMID: 37503272 Free PMC article. Updated. Preprint.
Similar articles
-
Hierarchical design of pseudosymmetric protein nanoparticles.Res Sq [Preprint]. 2023 Jul 10:rs.3.rs-3074553. doi: 10.21203/rs.3.rs-3074553/v1. Res Sq. 2023. Update in: Nature. 2025 Feb;638(8050):553-561. doi: 10.1038/s41586-024-08360-6. PMID: 37503272 Free PMC article. Updated. Preprint.
-
Hierarchical design of pseudosymmetric protein nanoparticles.bioRxiv [Preprint]. 2023 Jun 17:2023.06.16.545393. doi: 10.1101/2023.06.16.545393. bioRxiv. 2023. Update in: Nature. 2025 Feb;638(8050):553-561. doi: 10.1038/s41586-024-08360-6. PMID: 37398374 Free PMC article. Updated. Preprint.
-
Accurate design of co-assembling multi-component protein nanomaterials.Nature. 2014 Jun 5;510(7503):103-8. doi: 10.1038/nature13404. Epub 2014 May 25. Nature. 2014. PMID: 24870237 Free PMC article.
-
Practical approaches to designing novel protein assemblies.Curr Opin Struct Biol. 2013 Aug;23(4):632-8. doi: 10.1016/j.sbi.2013.06.002. Epub 2013 Jul 1. Curr Opin Struct Biol. 2013. PMID: 23827813 Review.
-
Nanoreactor Design Based on Self-Assembling Protein Nanocages.Int J Mol Sci. 2019 Jan 30;20(3):592. doi: 10.3390/ijms20030592. Int J Mol Sci. 2019. PMID: 30704048 Free PMC article. Review.
Cited by
-
Protein nanocages: A new frontier in mucosal vaccine delivery and immune activation.Hum Vaccin Immunother. 2025 Dec;21(1):2492906. doi: 10.1080/21645515.2025.2492906. Epub 2025 May 12. Hum Vaccin Immunother. 2025. PMID: 40353600 Free PMC article. Review.
-
Computational design of bifaceted protein nanomaterials.Nat Mater. 2025 Jul 31. doi: 10.1038/s41563-025-02295-7. Online ahead of print. Nat Mater. 2025. PMID: 40745094
-
Controlling nanocage assembly, towards developing a one-health "plug & play" platform for targeted therapy.Chem Commun (Camb). 2025 Aug 18. doi: 10.1039/d5cc03592a. Online ahead of print. Chem Commun (Camb). 2025. PMID: 40824119 Free PMC article. Review.
-
Four-component protein nanocages designed by programmed symmetry breaking.Nature. 2025 Feb;638(8050):546-552. doi: 10.1038/s41586-024-07814-1. Epub 2024 Dec 18. Nature. 2025. PMID: 39695226 Free PMC article.
-
Embracing Complexity: Peptides as Tunable Scaffolds in the Construction of Discrete Supramolecular Systems.Angew Chem Int Ed Engl. 2025 Aug 18;64(34):e202512014. doi: 10.1002/anie.202512014. Epub 2025 Jul 25. Angew Chem Int Ed Engl. 2025. PMID: 40631436 Free PMC article. Review.
References
-
- Alberts, B. The cell as a collection of protein machines: preparing the next generation of molecular biologists. Cell92, 291–294 (1998). - PubMed
-
- Goodsell, D. S. & Olson, A. J. Structural symmetry and protein function. Annu. Rev. Biophys. Biomol. Struct.29, 105–153 (2000). - PubMed
-
- King, N. P. & Lai, Y.-T. Practical approaches to designing novel protein assemblies. Curr. Opin. Struct. Biol.23, 632–638 (2013). - PubMed
-
- Douglas, T. & Young, M. Viruses: making friends with old foes. Science312, 873–875 (2006). - PubMed
-
- Howorka, S. Rationally engineering natural protein assemblies in nanobiotechnology. Curr. Opin. Biotechnol.22, 485–491 (2011). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

