Synaptic basis of feature selectivity in hippocampal neurons
- PMID: 39695232
- PMCID: PMC11988941
- DOI: 10.1038/s41586-024-08325-9
Synaptic basis of feature selectivity in hippocampal neurons
Abstract
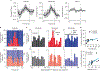
A central question in neuroscience is how synaptic plasticity shapes the feature selectivity of neurons in behaving animals1. Hippocampal CA1 pyramidal neurons display one of the most striking forms of feature selectivity by forming spatially and contextually selective receptive fields called place fields, which serve as a model for studying the synaptic basis of learning and memory. Various forms of synaptic plasticity have been proposed as cellular substrates for the emergence of place fields. However, despite decades of work, our understanding of how synaptic plasticity underlies place-field formation and memory encoding remains limited, largely due to a shortage of tools and technical challenges associated with the visualization of synaptic plasticity at the single-neuron resolution in awake behaving animals. To address this, we developed an all-optical approach to monitor the spatiotemporal tuning and synaptic weight changes of dendritic spines before and after the induction of a place field in single CA1 pyramidal neurons during spatial navigation. We identified a temporally asymmetric synaptic plasticity kernel resulting from bidirectional modifications of synaptic weights around the induction of a place field. Our work identified compartment-specific differences in the magnitude and temporal expression of synaptic plasticity between basal dendrites and oblique dendrites. Our results provide experimental evidence linking synaptic plasticity to the rapid emergence of spatial selectivity in hippocampal neurons, a critical prerequisite for episodic memory.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


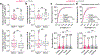

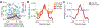




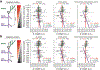




References
-
- Magee JC & Grienberger C Synaptic plasticity forms and functions. Annu. Rev. Neurosci 43, 95–117 (2020). - PubMed
-
- Bliss TV & Collingridge GL A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993). - PubMed
-
- Malenka RC & Bear MF LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 (2004). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

