Pediatric SARS-CoV-2 long term outcomes study (PECOS): cross sectional analysis at baseline
- PMID: 39695262
- PMCID: PMC12174577
- DOI: 10.1038/s41390-024-03777-1
Pediatric SARS-CoV-2 long term outcomes study (PECOS): cross sectional analysis at baseline
Abstract
Background: PECOS is an ongoing study aimed to characterize long-term outcomes following pediatric SARS-CoV-2 infection.
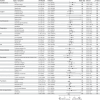
Methods: This is a cross-sectional analysis of infected and uninfected cohorts at baseline. Participants (0-21 years) with laboratory-confirmed SARS-CoV-2 infection were enrolled as infected. Uninfected were defined as individuals without history or laboratory evidence of SARS-CoV-2 infection. Outcome measures included demographics, medical history, review of symptoms, physical exam, cardiopulmonary evaluation and validated psychological and developmental surveys. Primary outcomes were cohort comparisons for abnormalities on all measures.
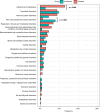
Results: 654 participants (541 infected, 113 uninfected) completed baseline visits by June 30, 2023. Infected participants were more likely to report constitutional (OR: 2.24), HEENT (OR: 3.74); respiratory (OR: 2.41), or gastrointestinal (OR: 2.58) symptoms. Infected had worse scores in domains of Pain, Fatigue, Global Health, Physical and Cognitive functioning, Mobility and Sleep disturbances when compared to uninfected controls using Patient Reported Outcomes. Cardiopulmonary findings were similar among cohorts.
Conclusions: The first report of this ongoing longitudinal study demonstrates that infected participants were more likely to report symptoms compared to uninfected controls, which may affect performance and quality of life of these individuals. Longitudinal data will increase understanding of long-term effects of SARS-CoV-2 infection in children.
Clinicaltrials: gov Identifier: NCT04830852 IMPACT: This study establishes a large, diverse, prospective, longitudinal, multi-center cohort of children with history of SARS-CoV-2 infection compared to an uninfected cohort to be followed for 3 years. Cross-sectional cohort analysis at study entry showed infected participants were more likely to report constitutional, respiratory, and GI symptoms compared to uninfected controls. Infected participants were more likely to have significantly worse parent-reported performance in 6 of 10 Patient Reported Outcome Measures domains. Continued study of this cohort will help identify clinical sequelae of COVID-19, characterize the immune response to SARS-CoV-2 infection, and identify potential genetic/immunologic factors associated with long-term outcomes.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare no competing interests. Informed consent: Written Informed Consent was obtained from all participants ≥18 years of age and at least one parent or legal guardian per participant <18 years of age. Written Assent was obtained for all participants 12–17 years of age.
Figures

References
-
- Organization, W. H. Who Covid-19 Dashboard, https://data.who.int/dashboards/covid19/cases (2024).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

