Evaluation of N-NOSE as a surveillance tool for recurrence in gastric and esophageal cancers: a prospective cohort study
- PMID: 39695429
- PMCID: PMC11656990
- DOI: 10.1186/s12885-024-13327-x
Evaluation of N-NOSE as a surveillance tool for recurrence in gastric and esophageal cancers: a prospective cohort study
Erratum in
-
Correction: Evaluation of N-NOSE as a surveillance tool for recurrence in gastric and esophageal cancers: a prospective cohort study.BMC Cancer. 2025 Jan 9;25(1):54. doi: 10.1186/s12885-025-13451-2. BMC Cancer. 2025. PMID: 39789462 Free PMC article. No abstract available.
Abstract
Objective: Early detection of recurrent gastric and esophageal cancers remains a critical challenge. Innovative and non-invasive cancer screening technologies, such as N-NOSE, can improve early detection. N-NOSE is a urine-based scent test that leverages the olfactory abilities of the nematode C. elegans. For the first time, this prospective study evaluates the efficacy of the N-NOSE chemotaxis index as a novel biomarker for postoperative surveillance and recurrence in patients with upper gastrointestinal cancers.
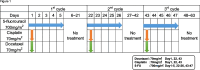
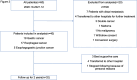
Methods: A two-year prospective cohort study was conducted at The University of Tokyo Hospital, involving 40 patients with gastric and esophageal cancers. Urine samples were collected pre- and postoperatively and analysed using the N-NOSE technique.
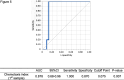
Results: In cases of recurrence with vascular invasion, the chemotaxis index at 100-fold urine dilution was significantly elevated compared to the non-recurrence group.
Conclusion: This study suggests the potential of N-NOSE as an effective follow-up tool for patients with upper gastrointestinal cancer, particularly those with vascular invasion. While N-NOSE has been validated to distinguish between cancer and non-cancer, and its performance compared to traditional markers has been proven, it has not been studied for recurrence. Our data highlights, for the first time, the capability of N-NOSE in the surveillance of cancer recurrence.
Keywords: Caenorhabditis elegans (C. elegans); Gastric cancer; Nematode-nose (N-NOSE); Oesophageal cancer; Urine.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures followed in this study were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the World Medical Association Declaration of Helsinki. The institutional Ethical Review Board of the University of Tokyo Hospital approved this study (2020122NI). This study was registered with UMIN (UMIN000042740). Informed consent statement: We obtained written informed consent from all patients prior to all study-related procedures in this study. Consent for publication: Not applicable. Competing interests: T.H. is the CEO and founder of Hirotsu Bio Science Inc.; E.d.L., H.H., M.M., and U.U. are Hirotsu Bio Science Inc. employees.
Figures



References
-
- Toh Y, Oki E, Minami K, Okamura T. Follow-up and recurrence after a curative esophagectomy for patients with esophageal cancer: the first indicators for recurrence and their prognostic values. Esophagus. 2010;7:37–43. - DOI
-
- Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, van der Sangen MJ, Beukema JC, Rütten H, Spruit PH, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. 2014;32(5):385–91. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

