The health-related quality of life in patients with dry eye syndrome: a cross-sectional study in Thailand
- PMID: 39695504
- PMCID: PMC11658330
- DOI: 10.1186/s12886-024-03808-9
The health-related quality of life in patients with dry eye syndrome: a cross-sectional study in Thailand
Abstract
Background: Dry eye syndrome (DES) is common but lack of data in quality of life (QoL) of DES patients in Thailand. The primary outcome of this study was to determine QoL and health utility in patients of DES by EuroQol 5-domain (EQ-5D) of the 5-level version (5 L) instrument. The secondary outcome was comparison of the utility in the patients of DES classified by severity and causes including the autoimmune and non-autoimmune diseases.
Method: The study was a cross-sectional study at a hospital in the northern part of Thailand. The inclusions DES patients were followed by Tear Film and Ocular surface Society the Dry Eye WorkShop II definition. The EQ-5D-5 L (Thai version) descriptive system and the EQ visual analogue scale (VAS) was instrument for QoL evaluation.
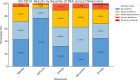
Result: Total patients of DES were fifty-six. The most patients were female. The mean age was 57.7(± 13.9) years. The mean of EQ-5D-utility and EQ-VAS were 0.76 (± 0.18) and 72.56 (± 15.19), respectively. The mean of EQ-5D-utility in these patients who were classified by severity including mild, moderate and severe were 0.84 (± 0.16), 0.78 (± 0.14) and 0.71 (± 0.22), respectively. There is no statistic significant in the EQ-5D-utility and EQ-VAS among severity and the causes of these patients.
Conclusions: This study demonstrated the importance of assessing QoL in DES. The EQ-5D-utility was accorded with the severity of DES. However, no statistic significant was showed in the mean of EQ-5D-utility and EQ-VAS between the severity and between the causes including the autoimmune and non-autoimmune diseases of these patients.
Keywords: Autoimmune; Dry eye syndrome; EQ-5D-5L; Quality of life; Utility.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Ethical approval was achieved by the Naresuan University Institutional Review Board and Ethics Committee (IRB No. P3-0041/2565). The Ethics Committees were in accordance with the Declaration of Helsinki. Consent to participate: Informed consent was obtained from all including dry eye syndrome patients. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources