Differential late-stage face processing in autism: a magnetoencephalographic study of fusiform gyrus activation
- PMID: 39695511
- PMCID: PMC11653706
- DOI: 10.1186/s12888-024-06400-z
Differential late-stage face processing in autism: a magnetoencephalographic study of fusiform gyrus activation
Abstract
Background: Autism is associated with alterations of social communication, such as during face-to-face interactions. This study aimed to probe face processing in autistics with normal IQ utilizing magnetoencephalography to examine event-related fields within the fusiform gyrus during face perception.
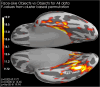
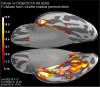
Methods: A case-control cohort of 22 individuals diagnosed with autism and 20 age-matched controls (all male, age 29.3 ± 6.9 years) underwent magnetoencephalographic scanning during an active task while observing neutral faces, face-like pareidolic objects, and non-face objects. The fusiform face area was identified using a face localizer for each participant, and the cortical activation pattern was normalized onto an average brain for subsequent analysis.
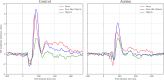
Results: Early post-stimulus activation amplitudes (before 100-200 ms) indicated differentiation between stimuli containing fundamental facial features and non-face objects in both groups. In contrast, later activation (400-550 ms) differentiated real faces from both pareidolic and non-face objects across both groups and faces from objects in controls but not in autistics. There was no effect of autistic-like traits.
Conclusions: The absence of group differences in early activation suggest intact face detection in autistics possessing a normal IQ. Later activation captures a greater degree of the complexity and social information from actual faces. Although both groups distinguished faces from pareidolic and non-face objects, the control group exhibited a slightly heightened differentiation at this latency, indicating a potential disadvantage for autistics in real face processing. The subtle difference in late-stage face processing observed in autistic individuals may reflect specific cognitive mechanisms related to face perception in autism.
Keywords: Autism; Biomarker; Face processing; Fusiform face area; Magnetoencephalography; Pareidolia.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Regional Ethical Board in Gothenburg, Sweden (DNR: 552–14) and informed consent was gathered from all participants. Consent for publication: The face stimuli used in the study are part of the openly available NimStim dataset of faces (Tottenham et al. 2009). We have received authorization by the authors to use the right-hand images in Fig. 1 (identity #28 in the dataset) for publication. Competing interests: The authors declare no competing interests.
Figures







References
-
- Farashi S, Jenabi E, Bashirian S, et al. Differences Between Autism Spectrum Disorder and Typically Developing Individuals During Visual Information Processing—A Systematic Review and Meta-analysis Focusing on Visual Event-Related Potentials. Rev J Autism Dev Disord. 2023. 10.1007/s40489-023-00383-5.
MeSH terms
LinkOut - more resources
Full Text Sources

