Supaglutide alleviates hepatic steatosis in monkeys with spontaneous MASH
- PMID: 39695722
- PMCID: PMC11658388
- DOI: 10.1186/s13098-024-01513-7
Supaglutide alleviates hepatic steatosis in monkeys with spontaneous MASH
Abstract
Background: Glucagon-like peptide 1 (GLP-1) is an incretin hormone and plays an important role in regulating glucose homeostasis. GLP-1 has a short half-life due to degrading enzyme dipeptidyl peptidase-IV and rapid kidney clearance, which limits its clinical application as a therapeutic agent. We demonstrated previously that supaglutide, a novel long-acting GLP-1 analog, exerted hypoglycemic, hypolipidemic, and weight loss effects in type 2 diabetic db/db mice, DIO mice, and diabetic monkeys. In the present study, we investigated supaglutide's therapeutic efficacy in rhesus monkeys with spontaneous metabolic dysfunction-associated steatohepatitis (MASH).
Methods: 15 rhesus monkeys with biopsy-confirmed MASH were divided into three groups, receiving supaglutide 50 µg/kg, supaglutide 150 µg/kg, and placebo, respectively, by weekly subcutaneous injection for 3 months. Liver fat content quantified by magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF), liver pathology, and metabolic parameters were assessed.
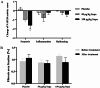
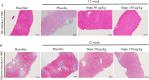
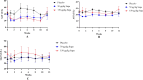
Results: We found that once-weekly subcutaneous injections of supaglutide for 3 months significantly reduced hepatic fat accumulation, with a 40% percentage decrease in MRI-PDFF from baseline (P < 0.001 vs. Placebo). Treatment with supaglutide alleviated hepatic histological steatosis (nonalcoholic fatty liver disease activity score P < 0.001 vs. Placebo) without worsening of fibrosis, as assessed by ultrasound-guided liver biopsy. Supaglutide concomitantly ameliorated liver injury exemplified by a lowering tendency of hepatic alanine aminotransferase levels. Supaglutide also decreased body weight in a dose-dependent fashion accompanied by decreased food intake, improved lipid profile and glycemic control.
Conclusions: Supaglutide exerts beneficial effects on hepatic and metabolic outcomes in spontaneous MASH monkeys.
Keywords: Glucagon-like peptide 1; Liver biopsy; Liver fat content; Metabolic dysfunction-associated steatohepatitis; Supaglutide.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Sichuan Primed Shines Bio-tech Co., Ltd (No. AW2110). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Novel GLP-1 analog supaglutide improves glucose homeostasis in diabetic monkeys.J Endocrinol. 2021 Feb;248(2):145-154. doi: 10.1530/JOE-20-0255. J Endocrinol. 2021. PMID: 33258802
-
Novel GLP-1 Analog Supaglutide Reduces HFD-Induced Obesity Associated with Increased Ucp-1 in White Adipose Tissue in Mice.Front Physiol. 2017 May 15;8:294. doi: 10.3389/fphys.2017.00294. eCollection 2017. Front Physiol. 2017. PMID: 28555111 Free PMC article.
-
Novel GLP-1 Analog Supaglutide Stimulates Insulin Secretion in Mouse and Human Islet Beta-Cells and Improves Glucose Homeostasis in Diabetic Mice.Front Physiol. 2019 Jul 25;10:930. doi: 10.3389/fphys.2019.00930. eCollection 2019. Front Physiol. 2019. PMID: 31404283 Free PMC article.
-
The role of glucagon-like peptide-1 receptor agonists in metabolic dysfunction-associated steatohepatitis.Diabetes Obes Metab. 2024 Jun;26(6):2001-2016. doi: 10.1111/dom.15524. Epub 2024 Mar 21. Diabetes Obes Metab. 2024. PMID: 38511418 Review.
-
Incretin-based investigational therapies for the treatment of MASLD/MASH.Diabetes Res Clin Pract. 2024 May;211:111675. doi: 10.1016/j.diabres.2024.111675. Epub 2024 Apr 16. Diabetes Res Clin Pract. 2024. PMID: 38636848 Review.
References
-
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–23. - PubMed
-
- Diehl AM, Day C. Cause, Pathogenesis, and treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377(21):2063–72. - PubMed
-
- Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589–600. - PubMed
-
- Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61(12):2461–98. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

