Therapeutic potential of antibody-drug conjugates possessing bifunctional anti-inflammatory action in the pathogenies of rheumatoid arthritis
- PMID: 39695738
- PMCID: PMC11656801
- DOI: 10.1186/s13075-024-03452-0
Therapeutic potential of antibody-drug conjugates possessing bifunctional anti-inflammatory action in the pathogenies of rheumatoid arthritis
Abstract
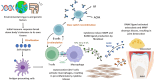
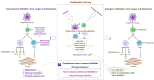
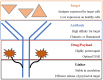
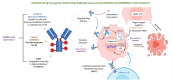
In an age where there is a remarkable upsurge in developing precision medicines, antibody-drug conjugates (ADCs) have emerged as a progressive therapeutic strategy. ADCs typically consist of monoclonal antibodies (mAb) conjugated to the cytotoxic payloads by utilizing a linker, combining the benefits of definitive target specificity of mAbs and potent killing impact of payload to achieve precise and efficient elimination of target cells. In addition to their well-established role in oncology, ADCs are currently demonstrating encouraging potential in addressing the unmet requirements in the treatment of autoimmune conditions such as rheumatoid arthritis (RA). Prevalent long-term autoimmune disease RA costs billions of dollars annually but still, there is a lack of precision-targeted therapeutics with minimal side effects. This review provides an overview of the RA pathogenesis, pre-existing therapies, and their limitations, the introduction of ADCs in RA treatment, the mechanism of ADCs, and a summary of ADCs in preclinical and clinical trials. Based on the literature we also propose a strategy in ADC synthesis, which may increase the efficiency in targeting multifactorial diseases like RA. We propose to utilize DMARDs (Disease-modifying anti-rheumatic drugs), the first-line treatment for RA, as a payload for ADC synthesis. DMARDs are the only class of medication that limits the disease progression, but their efficacy is limited due to off-target toxicities. Hence, utilizing them as payload will help to deliver them directly at the targeted site, reducing their off-target toxicity, which in turn will increase their efficiency in targeting disease. Also, as mAbs are not sufficient to achieve remission, they are given in combinations with DMARDs. Hence, synthesizing ADCs may reduce the multiple and higher dosages given to patients, which in turn may enhance patient compliance.
Keywords: Antibody-drug conjugates; Inflammation; Monoclonal antibodies; Disease modifying anti-rheumatoid drugs; Immunomodulation; Rheumatoid arthritis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All the authors know the content and agreed for publication. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Precision Medicine in Rheumatic Diseases: Unlocking the Potential of Antibody-Drug Conjugates.Pharmacol Rev. 2024 Jun 14;76(4):579-598. doi: 10.1124/pharmrev.123.001084. Pharmacol Rev. 2024. PMID: 38622001 Review.
-
Dual-payload antibody-drug conjugates: Taking a dual shot.Eur J Med Chem. 2025 Jan 5;281:116995. doi: 10.1016/j.ejmech.2024.116995. Epub 2024 Oct 23. Eur J Med Chem. 2025. PMID: 39481229 Review.
-
In vitro and in vivo evaluation of cysteine and site specific conjugated herceptin antibody-drug conjugates.PLoS One. 2014 Jan 14;9(1):e83865. doi: 10.1371/journal.pone.0083865. eCollection 2014. PLoS One. 2014. PMID: 24454709 Free PMC article.
-
Antibody-Drug Conjugates (ADCs): current and future biopharmaceuticals.J Hematol Oncol. 2025 Apr 30;18(1):51. doi: 10.1186/s13045-025-01704-3. J Hematol Oncol. 2025. PMID: 40307936 Free PMC article. Review.
-
Antibody-drug conjugates: Principles and opportunities.Life Sci. 2024 Jun 15;347:122676. doi: 10.1016/j.lfs.2024.122676. Epub 2024 Apr 28. Life Sci. 2024. PMID: 38688384 Review.
Cited by
-
Revitalizing Colchicine: Novel Delivery Platforms and Derivatives to Expand Its Therapeutic Potential.Int J Mol Sci. 2025 Aug 6;26(15):7591. doi: 10.3390/ijms26157591. Int J Mol Sci. 2025. PMID: 40806717 Free PMC article. Review.
References
-
- Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. 2021;41(5):863–77. - PubMed
-
- Finckh A, Gilbert B, Hodkinson B, Bae SC, Thomas R, Deane KD, Alpizar-Rodriguez D, Lauper K. Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol. 2022;18(10):591–602. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

