Alterations of the IKZF1-IKZF2 tandem in immune cells of schizophrenia patients regulate associated phenotypes
- PMID: 39695786
- PMCID: PMC11658472
- DOI: 10.1186/s12974-024-03320-3
Alterations of the IKZF1-IKZF2 tandem in immune cells of schizophrenia patients regulate associated phenotypes
Abstract
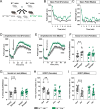
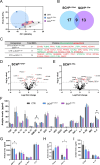
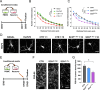
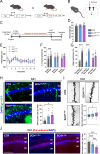
Schizophrenia is a complex multifactorial disorder and increasing evidence suggests the involvement of immune dysregulations in its pathogenesis. We observed that IKZF1 and IKZF2, classic immune-related transcription factors (TFs), were both downregulated in patients' peripheral blood mononuclear cells (PBMCs) but not in their brain. We generated a new mutant mouse model with a reduction in Ikzf1 and Ikzf2 to study the impact of those changes. Such mice developed deficits in the three dimensions (positive-negative-cognitive) of schizophrenia-like phenotypes associated with alterations in structural synaptic plasticity. We then studied the secretomes of cultured PBMCs obtained from patients and identified potentially secreted molecules, which depended on IKZF1 and IKZF2 mRNA levels, and that in turn have an impact on neural synchrony, structural synaptic plasticity and schizophrenia-like symptoms in in vivo and in vitro models. Our results point out that IKZF1-IKZF2-dependent immune signals negatively impact on essential neural circuits involved in schizophrenia.
Keywords: CCL5; CXCL10; Cognitive symptoms; Helios; IL-4; Ikaros; Lymphocytes; Mouse models; Negative symptoms; Neuronal networks.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All the procedures for the obtention of post-mortem samples followed the ethical guidelines of the Declaration of Helsinki and local ethical committees (Universitat de Barcelona: IRB00003099; Fundació CEIC Sant Joan de Déu: BTN-PSSJD). The blood samples of this study (N◦PI17/00246, PI Belen Arranz) was recruited in the Outpatient clinic located in Cornellà, Barcelona, Spain (Parc Sanitari Sant Joan de Deu). All animal procedures were approved by local committees [Universitat de Barcelona, CEEA (136/19); Generalitat de Catalunya (DAAM 10786) following the European Communities Council Directive (86/609/EU). Competing interests: The authors declare no competing interests.
Figures



References
-
- Aguilar R, Campo JJ, Chicuecue S, Cisteró P, Català A, Luis L, Ubillos I, Galatas B, Aide P, Guinovart C, Moncunill G, Dobaño C. Changing plasma cytokine, chemokine and growth factor profiles upon differing malaria transmission intensities. Malar J. 2019;18:406. 10.1186/s12936-019-3038-x. - PMC - PubMed
-
- Ahmad SF, Nadeem A, Ansari MA, Bakheet SA, AL-Ayadhi LY, Attia SM. Downregulation in Helios transcription factor signaling is associated with immune dysfunction in blood leukocytes of autistic children. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;85:98–104. 10.1016/j.pnpbp.2018.04.011. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

