Prognostic feature based on androgen-responsive genes in bladder cancer and screening for potential targeted drugs
- PMID: 39695796
- PMCID: PMC11657289
- DOI: 10.1186/s13040-024-00377-x
Prognostic feature based on androgen-responsive genes in bladder cancer and screening for potential targeted drugs
Abstract
Objectives: Bladder cancer (BLCA) is a tumor that affects men more than women. The biological function and prognostic value of androgen-responsive genes (ARGs) in BLCA are currently unknown. To address this, we established an androgen signature to determine the prognosis of BLCA.
Methods: Sequencing data for BLCA from the TCGA and GEO datasets were used for research. The tumor microenvironment (TME) was measured using Cibersort and ssGSEA. Prognosis-related genes were identified and a risk score model was constructed using univariate Cox regression, LASSO regression, and multivariate Cox regression. Drug sensitivity analysis was performed using Genomics of drug sensitivity in cancer (GDSC). Real-time quantitative PCR was performed to assess the expression of representative genes in clinical samples.
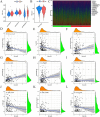
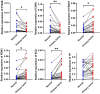
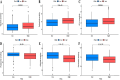
Results: ARGs (especially the CDK6, FADS1, PGM3, SCD, PTK2B, and TPD52) might regulate the progression of BLCA. The different expression patterns of ARGs may lead to different immune cell infiltration. The risk model indicates that patients with higher risk scores have a poorer prognosis, more stromal infiltration, and an enrichment of biological functions. Single-cell RNA analysis, bulk RNA data, and PCR analysis support the reliability of this risk model, and a nomogram was also established for clinical use. Drug prediction analysis showed that high-risk patients had a better response to fludarabine, AZD8186, and carmustine.
Conclusion: ARGs played an important role in the progression, immune infiltration, and prognosis of BLCA. The ARGs model has high accuracy in predicting the prognosis of BLCA patients and provides more effective medication guidelines.
Keywords: Androgen-responsive genes; Bladder cancer; Drug sensitivity; Immune cell infiltration; Men; Prognosis; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Committee of the Affiliated Hospital of Guangdong Medical of China (Approval No. YJYS2023179) and was conducted following the Declaration of Helsinki. Written informed consent was obtained from individual or guardian participants. Consent for publication: All authors have read and agreed to the published version of the manuscript. Competing interests: The authors declare no competing interests.
Figures






References
-
- Lenis AT, Lec PM, Chamie K, et al. Bladder Cancer: Rev JAMA. 2020;324(19):1980–91. - PubMed
-
- Lobo N, Shariat SF, Guo CC, et al. What is the significance of variant histology in Urothelial Carcinoma? Eur Urol Focus. 2020;6(4):653–63. - PubMed
-
- Cai Z, Liu Q. Understanding the Global Cancer statistics 2018: implications for cancer control. Sci China Life Sci. 2021;64(6):1017–20. - PubMed
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Cumberbatch MG, Cox A, Teare D, et al. Contemporary occupational carcinogen exposure and bladder Cancer: a systematic review and Meta-analysis. JAMA Oncol. 2015;1(9):1282–90. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

