Regulation of injury-induced skeletal myofiber regeneration by glucose transporter 4 (GLUT4)
- PMID: 39695900
- PMCID: PMC11656879
- DOI: 10.1186/s13395-024-00366-y
Regulation of injury-induced skeletal myofiber regeneration by glucose transporter 4 (GLUT4)
Abstract
Background: Insulin resistance and type 2 diabetes impair cellular regeneration in multiple tissues including skeletal muscle. The molecular basis for this impairment is largely unknown. Glucose uptake via glucose transporter GLUT4 is impaired in insulin resistance. In healthy muscle, acute injury stimulates glucose uptake. Whether decreased glucose uptake via GLUT4 impairs muscle regeneration is presently unknown. The goal of this study was to determine whether GLUT4 regulates muscle glucose uptake and/or regeneration following acute injury.
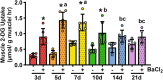
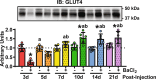
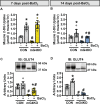
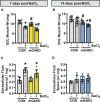
Methods: Tibialis anterior and extensor digitorum longus muscles from wild-type, control, or muscle-specific GLUT4 knockout (mG4KO) mice were injected with the myotoxin barium chloride to induce muscle injury. After 3, 5, 7, 10, 14, or 21 days (in wild-type mice), or after 7 or 14 days (in control & mG4KO) mice, muscles were isolated to examine [3H]-2-deoxyglucose uptake, GLUT4 levels, extracellular fluid space, fibrosis, myofiber cross-sectional area, and myofiber centralized nuclei.
Results: In wild-type mice, muscle glucose uptake was increased 3, 5, 7, and 10 days post-injury. There was a rapid decrease in GLUT4 protein levels that were restored to baseline at 5-7 days post-injury, followed by a super-compensation at 10-21 days. In mG4KO mice, there were no differences in muscle glucose uptake, extracellular fluid space, muscle fibrosis, myofiber cross-sectional areas, or percentage of centrally nucleated myofibers at 7 days post-injury. In contrast, at 14 days injured muscles from mG4KO mice exhibited decreased glucose uptake, muscle weight, myofiber cross sectional areas, and centrally nucleated myofibers, with no change in extracellular fluid space or fibrosis.
Conclusions: Collectively, these findings demonstrate that glucose uptake via GLUT4 regulates skeletal myofiber regeneration following acute injury.
Keywords: Barium chloride; Diabetes mellitus type 2; Extracellular fluid; Fibrosis; Glucose; Glucose transporter type 4; Insulin resistance; Knockout; Mice; Muscle; Regeneration.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Procedures were performed in accordance with the Indiana University School of Medicine Institutional Animal Care and Use Committee (IACUC, protocol# 21053 approved on 17 May 2021 and protocol# 21118 approved on 31 August 2021), and the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988;255:E769–74. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

