Aspergillus nidulans cell wall integrity kinase, MpkA, impacts cellular phenotypes that alter mycelial-material mechanical properties
- PMID: 39695906
- PMCID: PMC11658146
- DOI: 10.1186/s40694-024-00191-4
Aspergillus nidulans cell wall integrity kinase, MpkA, impacts cellular phenotypes that alter mycelial-material mechanical properties
Abstract
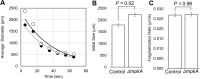
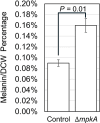
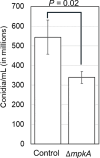
Mycelial materials are an emerging, natural material made from filamentous fungi that have the potential to replace unsustainable materials used in numerous commercial applications (e.g., packaging, textiles, construction). Efforts to change the mechanical properties of mycelial-materials have typically involved altering growth medium, processing approaches, or fungal species. Although these efforts have shown varying levels of success, all approaches have shown there is a strong correlation between phenotype (of both fungal mycelia and mycelial material's assembly) and resultant mechanical properties. We hypothesize that genetic means can be used to generate specific fungal phenotypes, leading to mycelial materials with specific mechanical properties. To begin to test this hypothesis, we used a mutant of the model filamentous fungus, Aspergillus nidulans, with a deletion in the gene encoding the last kinase in the cell wall integrity (CWI) signaling pathway, mpkA. We generated one set of mycelial materials from the ΔmpkA deletion mutant (A1404), and another from its isogenic parent (A1405; control). When subjected to tensile testing, and compared to material generated from the control, ΔmpkA material has similar elastic modulus, but significantly increased ultimate tensile strength, and strain at failure. When subjected to a fragmentation assay (i.e., resistance to shear-stress), the ΔmpkA material also had higher relative mechanical strength. To determine possible causes for this behavior, we carried out a comprehensive set of phenotype assessments focused on: three-dimensional structure, hyphal morphology, hyphal growth behaviors, and conidial development. We found, compared to the control, material generated from the ΔmpkA mutant manifests significantly less development, a modified cell wall composition, larger diameter hyphae, more total biomass, higher water capacity and more densely packed material, which all appear to impact the altered mechanical properties.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures






References
-
- Ramesh M, Palanikumar K, Reddy KH. Plant fibre based bio-composites: sustainable and renewable green materials. Renew Sustain Energy Rev. 2017;79:558–84. - DOI
-
- Nakhate P, Van Der Meer Y. A systematic review on seaweed functionality: a sustainable bio-based material. Sustainability. 2021;13(11):6174. - DOI
-
- Gawel E, Pannicke N, Hagemann N. A path transition towards a bioeconomy—the crucial role of sustainability. Sustainability. 2019;11(11):3005. - DOI
-
- Nations FaAOotU. Bioeconomy for a sustainable future. Food and Agriculture Organization of the United Nations; 2021.
Grants and funding
LinkOut - more resources
Full Text Sources

