CaV3.2 T-type calcium channels contribute to CGRP- induced allodynia in a rodent model of experimental migraine
- PMID: 39695919
- PMCID: PMC11656763
- DOI: 10.1186/s10194-024-01921-0
CaV3.2 T-type calcium channels contribute to CGRP- induced allodynia in a rodent model of experimental migraine
Abstract
Background: Migraine is a painful neurological syndrome characterized by attacks of throbbing headache, of moderate to severe intensity, which is associated with photo- and phono- sensitivity as well as nausea and vomiting. It affects about 15% of the world's population being 2-3 times more prevalent in females. The calcitonin gene-related peptide (CGRP) is a key mediator in the pathophysiology of migraine, and a significant advance in the field has been the development of anti-CGRP therapies. The trigeminal ganglion (TG) is thought to be an important site of action for these drugs. Moreover, experimental migraine can be induced by CGRP injection in the TG. The signaling pathway induced by CGRP in the TG is not fully understood, but studies suggest that voltage-gated calcium channels contribute to CGRP effects relevant to migraine.
Objective: We hypothesised that CGRP injection in the TG enhances CaV3.2 T-type calcium channel currents to contribute to the development of periorbital mechanical allodynia.
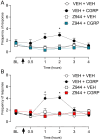
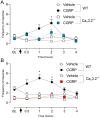
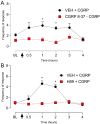
Results: A Co-Immunoprecipitation assay in tsA-201 cells revealed that CaV3.2 channels form a complex with RAMP-1, a component of the CGRP receptor. Constitutive CGRPR activity was able to inhibit CaV3.2 channels and induce a depolarizing shift in both activation and inactivation curves. Incubation of TG neurons with CGRP increased T-type current density by ~ 3.6 fold, an effect that was not observed in TG neurons from CaV3.2 knockout mice. Incubation of TG neurons with Z944, a pan T-type channel blocker, resulted in an approximately 80% inhibition of T-type currents. In vivo, this treatment abolished the development of periorbital mechanical allodynia induced by CGRP in male and female mice. Likewise, CaV3.2 knockout mice did not develop periorbital mechanical allodynia after intraganglionic CGRP injection. Finally, we demonstrated that the CGRP effect depends on the activation of its canonical GPCR, followed by protein kinase A activation.
Conclusion: The present study suggests that CGRP modulates CaV3.2 in the TG, an effect possibly mediated by the canonical CGRP receptor and PKA activation. The increase in T-type currents in the TG may represent a contributing factor for the initiation and maintenance of the headache pain during migraine.
Keywords: Calcitonin gene-related peptide; Electrophysiology; Mice; Periorbital mechanical allodynia; RAMP-1; Trigeminal ganglion; Voltage-gated calcium channels.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Headache Classification Committee of the International Headache Society (IHS) (2018) The International classification of Headache disorders, 3rd edition. Cephalalgia 38(1):1–211 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

