Effect of continuous infusion in alleviating pain during male urethral catheterization
- PMID: 39695957
- PMCID: PMC11657273
- DOI: 10.1186/s12871-024-02848-4
Effect of continuous infusion in alleviating pain during male urethral catheterization
Abstract
Aims: The aim of this study was to explore whether continuous infusion causing lubrication can effectively alleviate pain during male urethral catheterization.
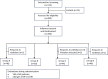
Methods: This prospective, multicenter, double-blinded study included 190 male patients scheduled for urethral catheterization. Patients were randomly allocated into four groups: Group A: the catheter was lubricated with paraffin; Group B: the catheter was lubricated with compound lidocaine gel; Group C: the pump continuously infusing with sterilized water; Group D: the pump continuously infusing with 2% lidocaine. The primary outcome was the visual analogue scale (VAS) scores. Statistical analysis system (SAS) (version 9.4) was used to perform all the statistical analyses. Significance for all results was set at P < 0.05.
Results: The VAS of Group D was the lowest (18.90 ± 11.44), followed by the Group C (33.00 ± 11.07), and the VAS of Group A was the highest (53.98 ± 14.76). There were significant differences in VAS in Group D compared to Group A(P < 0.0001), Group B(P < 0.0001) and Group C (P < 0.0001), Group C compared to Group A (P < 0.0001) and Group B(P < 0.0001), Group B compared to Group A (P < 0.0001), indicating that patients treated with lidocaine infusion (Group D) experienced significantly less pain than did those in the other three groups.
Conclusions: Continuous infusion with sterilized water during catheterization is an efficient method for lubricating the urethral mucosa; furthermore, infusion with 2% lidocaine provides better analgesia as well as lubrication.
Trial registration: The study protocol was registered in the Chinese Clinical Trial Registry (ChiCTR2300070866) ( https://www.chictr.org.cn/showproj.html?proj=194591 ) on Apr. 25th, 2023.
Keywords: Continuous infusion; Multicenter study; Pain; Urethral catheterization.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Clinical Research Ethics Committee of the Second Xiangya Hospital, Central South University. The trial was strictly designed in accordance with the CONSORT statement and Helsinki Declaration, and all the patients have signed the informed consent to participate the study before the procedure. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

