Comparing the protection of heterologous booster of inhaled Ad5-nCoV vaccine and hybrid immunity against Omicron BA.5 infection: a cohort study of hospital staff in China
- PMID: 39695978
- PMCID: PMC11654266
- DOI: 10.1186/s12879-024-10250-1
Comparing the protection of heterologous booster of inhaled Ad5-nCoV vaccine and hybrid immunity against Omicron BA.5 infection: a cohort study of hospital staff in China
Abstract
Background: After the exit "zero-COVID" strategy in mainland China by the end of 2022, a large-scale COVID-19 outbreak seeded by Omicron variants occurred. An inhaled adenovirus type-5 vector-based (i.e., inhaled Ad5-nCoV) COVID-19 vaccine was licensed earlier in 2021. In this study, we aimed to assess the real-world effectiveness of a heterologous booster of inhaled Ad5-nCoV vaccine against Omicron infection and compared with the protection from hybrid immunity (i.e., prior breakthrough infection).
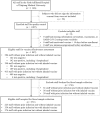
Methods: In this retrospective cohort study, we identified 1087 out of a total of 1146 hospital staff from a tertiary hospital in Urumqi city, China from November 22 to December 29, 2022. Demographic characteristics, baseline health status, occupation, behavioral factors, laboratory test of serological IgG antibody, and timeline from immunization to laboratory-testing outcome were obtained. We analysed the individual-level vaccination status of inhaled Ad5-nCoV vaccine, prior SARS-CoV-2 infection status and baseline vaccination status, and other risk factors before follow-up. The protective effects of the heterologous inhaled Ad5-nCoV vaccine and hybrid immunity against Omicron BA.5 infection and hospitalization were calculated as relative rate reduction (RRR), which was estimated using multivariate Poisson regression models.
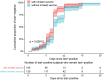
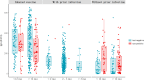
Results: A total of 1087 hospital staff (median age of 34 years, and 343 males [31.6%]), including 931 accepted for serological antibody tests, were recruited to assess the vaccine effectiveness (VE) of the inhaled Ad5-nCoV booster and hybrid immunity. Among the 1087 participants, 413 had a history of prior SARS-CoV-2 infection (before follow-up) but did not receive an inhaled Ad5-nCoV booster, and 674 reported no prior infection, including 390 who received an inhaled Ad5-nCoV booster. The highest serological IgG antibody level was detected among the inhaled Ad5-nCoV group, with a median of 294.59 S/CO, followed by the hybrid immunity group, with a median of 93.65 S/CO compared to the reference level of the inactivated vaccine group (most of whom received the Sinopharm/BBIBP-CorV vaccine). The inhaled Ad5-nCoV booster and hybrid immunity yielded RRRs of 41.9% (95% CI: 24.8, 55.0) and 97.9% (95% CI: 94.2, 99.2), respectively, against Omicron BA.5 infection, regardless of symptom status.
Conclusion: We found that hybrid immunity could provide a high level of protection against Omicron infection, while a heterologous inhaled Ad5-nCoV booster conferred a moderate level of protection. Our findings supported the rollout of a heterologous vaccination strategy regardless of preexisting vaccine coverage.
Keywords: Inhaled Ad5-nCoV vaccine; SARS-CoV-2; Serological IgG antibody; Vaccine effectiveness.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the institutional ethics committee of Xinjiang Medical University. Medical records were kept confidential in full at the Sixth Affiliated Hospital of Xinjiang Medical University, and the personal identity of the subjects was not and will not be disclosed in any report on the results of this study. Informed consent is obtained from all the participants. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- WHO. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. (https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1...., October 21, 2022).
-
- WHO. Weekly epidemiological update on COVID-19–19. October 2022. (https://www.who.int/publications/m/item/weekly-epidemiological-update-on..., October 19, 2022).
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

