Exploring the gut-brain axis in alzheimer's disease treatment via probiotics: evidence from animal studies-a systematic review and meta-analysis
- PMID: 39695988
- PMCID: PMC11657461
- DOI: 10.1186/s12883-024-03978-5
Exploring the gut-brain axis in alzheimer's disease treatment via probiotics: evidence from animal studies-a systematic review and meta-analysis
Abstract
Introduction: Alzheimer's disease (AD) is a prevalent neurodegenerative disorder in the elderly, causing cognitive impairment. Its pathogenesis is characterized by amyloid beta deposition, neurofibrillary tangles, and neuroinflammation. Recent research has identified the link between gut dysbiosis, an imbalance of intestinal microorganisms, to this pathogenesis via the gut-brain axis. This study aims to review the probiotics' therapeutic effect, targeting the gut-brain axis, for AD treatment in animals.
Methods: The method utilized in this study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. Three reviewers searched articles through PubMed, Scopus, and Embase using advanced search strategy. Articles published between 2010 and 2023 that met the criteria were included.
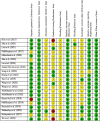
Results: Of 2,273 articles, 21 animal studies measuring the effects of probiotics genera Lactobacillus and/or Bifidobacterium on AD via at least one of these four outcomes: AD pathology, cognitive function, neuroinflammation, and gut microbiota composition. The results demonstrated that probiotics could repair gut dysbiosis by decreasing pro-inflammatory bacteria and increasing anti-inflammatory bacteria. Repaired dysbiosis was found to be associated with less neuroinflammation as significant reductions in neuroinflammatory markers related to the pathogenesis of AD such as TNF-α (SMD = -2.08, P = 0.005), IL-6 (SMD = -2.98, P < 0.0005), and IL-1β (SMD = -2.49, P = 0.003) were observed. Reduced amyloid beta deposition (SMD = -1.17, P = 0.009) was reported, but reduction in tau hyperphosphorylation was found to be insignificant. For cognitive function, positive results were demonstrated for all three aspects of cognitive function including long-term memory (SMD = 2.55, P < 0.00001), short-term memory (SMD = 1.32, P = 0.003), and spatial recognition (SMD = -1.13, P < 0.00001).
Conclusions: Particular formulas of probiotics showed potential effectiveness in AD therapies with demonstrated association with the gut-brain axis. Future studies are required to investigate strain-specific results and optimal dosages and regimens.
Keywords: Alzheimer’s disease; Gut microbiota; Gut-brain axis; Probiotics.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

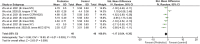


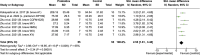

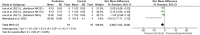
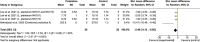
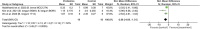



References
-
- Sheppard O, Coleman M. Alzheimer's disease: etiology, neuropathology and pathogenesis. In: Huang X, editor. Alzheimer's disease: drug discovery. Brisbane (AU): Exon Publications; 2020. - PubMed
-
- Centers for Disease Control and Prevention. About Alzheimer’s Disease. 2023. https://www.cdc.gov/aging/aginginfo/alzheimers.htm.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

