Impaired liver function: effect on paclitaxel toxicity, dose modifications and overall survival
- PMID: 39696046
- PMCID: PMC11658450
- DOI: 10.1186/s12885-024-13330-2
Impaired liver function: effect on paclitaxel toxicity, dose modifications and overall survival
Abstract
Background: The anticancer drug paclitaxel is primarily metabolized in the liver. Previous studies have indicated a correlation between impaired liver function and paclitaxel toxicity, which may indicate dose reduction. Since the evidence is limited, the aim of this study was to investigate the effect of impaired liver function on the hematological toxicity of paclitaxel, dose modifications and overall survival (OS).
Methods: For this single-center retrospective observational study, patients treated with paclitaxel for breast, esophageal and ovarian cancer at the University Medical Centre Utrecht between 2011 and 2022 were identified from the Utrecht Patient Oriented Database (UPOD). Based on regression analysis, the risk of developing Grade 3/4 hematological toxicity was compared between patients with normal and impaired (based on the NCI criteria for bilirubin and ASAT (aspartate aminotransferase) concentrations) liver function. Additionally, differences in the occurrence of toxicity-related dose modifications and OS were evaluated between the two groups.
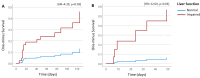
Results: A total of 569 patients were included. Breast cancer patients who were receiving advanced treatment and had mildly impaired liver function (ASAT ≤ 2x ULN, bilirubin ≤ ULN) had an increased risk of developing grade 3/4 neutropenia (HR = 4.39, 95% CI 1.20-16.02; p = 0.03). In addition, patients with impaired liver function treated according to the advanced ovarian cancer regimen had an increased risk of developing grade 3/4 leukopenia (HR = 12.64, 95% CI 2.12-75.22, p = 0.01) and dose modification (treatment discontinuation) (HR = 3.91, 95% CI 1.74-8.79, p < 0.01). Impaired liver function was also associated with decreased OS in inoperable esophageal and advanced ovarian cancer patients (HR = 7.65, 95% CI 2.54-23.1, p < 0.01 and HR = 2.98, 95% CI 1.36-6.54, p < 0.01, respectively). The risk of developing grade 3/4 hematological toxicity during lower-dose paclitaxel treatment protocols was not significantly different in patients with impaired liver function.
Conclusions: This study revealed that patients with impaired liver function treated with paclitaxel for breast and ovarian cancer in an advanced setting are at greater risk of developing hematological toxicity than patients with normal liver function at the start of therapy. Furthermore, in patients with ovarian (advanced) or inoperable esophageal cancer, impaired liver function is associated with decreased OS. Within these groups of patients, it is important to weigh the risk of upfront paclitaxel dose modifications versus an adaptive strategy.
Keywords: Impaired liver function; Paclitaxel toxicity; paclitaxel dose modifications.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The medical research ethics committee of UMC Utrecht (MREC Utrecht) confirmed that the Medical Research Involving Human Subjects Act (WMO) does not apply to this study. Therefore, official approval for this study under the WMO is not needed (see the attached file for the declaration by MREC Utrecht - protocol number 21–555/C). Consent for publication: N.A. Competing interests: The authors declare no competing interests.
Figures


References
-
- Ingelman-Sundberg M. Pharmacogenetics: an opportunity for a safer and more efficient pharmacotherapy. J Intern Med. 2001;250(3):186–200. - PubMed
-
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138(1):103–41. - PubMed
-
- Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64(12):1147–61. - PubMed
-
- Krens SD, Lassche G, Jansman FGA, Desar IME, Lankheet NAG, Burger DM, van Herpen CML, van Erp NP. Dose recommendations for anticancer drugs in patients with renal or hepatic impairment. Lancet Oncol. 2019;20(4):e200–7. - PubMed
-
- Giraud EL, de Lijster B, Krens SD, Desar IME, Boerrigter E, van Erp NP. Dose recommendations for anticancer drugs in patients with renal or hepatic impairment: an update. Lancet Oncol. 2023;24(6):e229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

