Aberrant amplitude of low-frequency fluctuation and functional connectivity in children with different subtypes of ADHD: a resting-state fNIRS study
- PMID: 39696119
- PMCID: PMC11658069
- DOI: 10.1186/s12888-024-06350-6
Aberrant amplitude of low-frequency fluctuation and functional connectivity in children with different subtypes of ADHD: a resting-state fNIRS study
Abstract
Background: Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder with different subtypes of pathogenesis. Insufficient research on the subtypes of ADHD has limited the effectiveness of therapeutic methods.
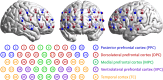
Methods: This study used resting-state functional near-infrared spectroscopy (fNIRS) to record hemodynamic signals in 34 children with ADHD-combined subtype (ADHD-C), 52 children with ADHD-inattentive subtype (ADHD-I), and 24 healthy controls (HCs). The amplitude of low-frequency fluctuation (ALFF) and the functional connectivity (FC) analysis were conducted for all subjects.
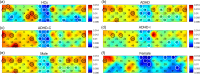
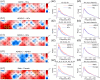
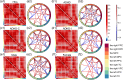
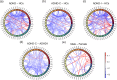
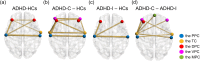
Results: Compared with HCs, the ADHD group exhibited significantly increased ALFF and decreased FC. The ADHD-C group showed significantly higher ALFF in partial brain regions and significantly lower FC between multiple brain regions than participants with ADHD-I. The male group displayed a significant increase in ALFF in some brain regions, while no significant difference was found in FC when compared to the female group.
Conclusions: This study provides evidence to support the subtype classification of ADHD-I and ADHD-C, and the combined analysis of ALFF and FC has the potential to be a promising biomarker for the diagnosis of ADHD.
Keywords: Amplitude of low-frequency fluctuation; Functional connectivity; Functional near-infrared spectroscopy; Subtypes of ADHD.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Medical Research Ethics Committee of Peking University Sixth Hospital (IRB number: 2016-15), in accordance with the "Ethical Review Measures for Life Sciences and Medical Research Involving Human Beings". Written informed consent was obtained from the guardians of all participants before the experiment. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Baggio S, Billieux J, Dirkzwager A, Iglesias K, Moschetti K, Perroud N, Schneider M, Vernaz N, Wolff H, Heller P. Protocol of a monocentric, double-blind, randomized, superiority, controlled trial evaluating the effect of in-prison OROS-methylphenidate vs. placebo treatment in detained people with attention-deficit hyperactivity disorder (BATIR). Trials. 2024;25(1):23. - PMC - PubMed
-
- Barra S, Turner D, Müller M, Hertz PG, Retz-Junginger P, Tüscher O, Huss M, Retz W. ADHD symptom profiles, intermittent explosive disorder, adverse childhood experiences, and internalizing/externalizing problems in young offenders. Eur Arch Psychiatry Clin NeuroSci. 2022;272(2):257–69. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

