ZDHHC20 mediated S-palmitoylation of fatty acid synthase (FASN) promotes hepatocarcinogenesis
- PMID: 39696259
- PMCID: PMC11657817
- DOI: 10.1186/s12943-024-02195-5
ZDHHC20 mediated S-palmitoylation of fatty acid synthase (FASN) promotes hepatocarcinogenesis
Abstract
Background: Protein palmitoylation is a reversible fatty acyl modification that undertakes important functions in multiple physiological processes. Dysregulated palmitoylations are frequently associated with the formation of cancer. How palmitoyltransferases for S-palmitoylation are involved in the occurrence and development of hepatocellular carcinoma (HCC) is largely unknown.
Methods: Chemical carcinogen diethylnitrosamine (DEN)-induced and DEN combined CCl4 HCC models were used in the zinc finger DHHC-type palmitoyltransferase 20 (ZDHHC20) knockout mice to investigate the role of ZDHHC20 in HCC tumourigenesis. Palmitoylation liquid chromatography-mass spectrometry analysis, acyl-biotin exchange assay, co-immunoprecipitation, ubiquitination assays, protein half-life assays and immunofluorescence microscopy were conducted to explore the downstream regulators and corresponding mechanisms of ZDHHC20 in HCC.
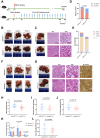
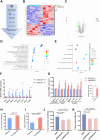
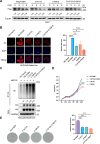
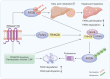
Results: Knocking out of ZDHHC20 significantly reduced hepatocarcinogenesis induced by chemical agents in the two HCC mouse models in vivo. 97 proteins with 123 cysteine sites were found to be palmitoylated in a ZDHHC20-dependent manner. Among these, fatty acid synthase (FASN) was palmitoylated at cysteines 1471 and 1881 by ZDHHC20. The genetic knockout or pharmacological inhibition of ZDHHC20, as well as the mutation of the critical cysteine sites of FASN (C1471S/C1881S) accelerated the degradation of FASN. Furthermore, ZDHHC20-mediated FASN palmitoylation competed against the ubiquitin-proteasome pathway via the E3 ubiquitin ligase complex SNX8-TRIM28.
Conclusions: Our findings demonstrate the critical role of ZDHHC20 in promoting hepatocarcinogenesis, and a mechanism underlying a mutual restricting mode for protein palmitoylation and ubiquitination modifications.
Keywords: FASN; Hepatocarcinogenesis; S-palmitoylation; ZDHHC20.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: All animal experiments including plans and protocols were reviewed and approved by the Stamp of The Animal Ethical and Welfare Committee of Tianjin Medical University Cancer Institute and Hospital (LLSP2019001). and Laboratory Animal Use Management and Welfare Ethics Committee of Chongqing University Cancer Hospital (CQCH-LAE-A0000202015). Human HCC samples used in this study was approved by the Chongqing University Cancer Hospital of Medicine Institutional Review Board. Competing interests: The authors declare no competing interests.
Figures




References
-
- Schmidt MF, Schlesinger MJ. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979;17(4):813–9. - PubMed
-
- Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8(1):74–84. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

