Numerical study of the effects of minor structures and mean velocity fields in the cerebrospinal fluid flow
- PMID: 39696350
- PMCID: PMC11657969
- DOI: 10.1186/s12987-024-00604-x
Numerical study of the effects of minor structures and mean velocity fields in the cerebrospinal fluid flow
Abstract
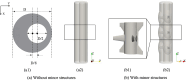
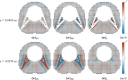
The importance of optimizing intrathecal drug delivery is highlighted by its potential to improve patient health outcomes. Findings from previous computational studies, based on an individual or a small group, may not be applicable to the wider population due to substantial geometric variability. Our study aims to circumvent this problem by evaluating an individual's cycle-averaged Lagrangian velocity field based on the geometry of their spinal subarachnoid space. It has been shown by Lawrence et al. (J Fluid Mech 861:679-720, 2019) that dominant physical mechanisms, such as steady streaming and Stokes drift, are key to facilitating mass transport within the spinal canal. In this study, we computationally modeled pulsatile cerebrospinal fluid flow fields and Lagrangian velocity field within the spinal subarachnoid space. Our findings highlight the essential role of minor structures, such as nerve roots, denticulate ligaments, and the wavy arachnoid membrane, in modulating flow and transport dynamics within the spinal subarachnoid space. We found that these structures can enhance fluid transport. We also emphasized the need for particle tracking in computational studies of mass transport within the spinal subarachnoid space. Our research illuminates the relationship between the geometry of the spinal canal and transport dynamics, characterized by a large upward cycle-averaged Lagrangian velocity zone in the wider region of the geometry, as opposed to a downward zone in the narrower region and areas close to the wall. This highlights the potential for optimizing intrathecal injection protocols by harnessing natural flow dynamics within the spinal canal.
Keywords: Cerebrospinal fluid; Computational fluid dynamics; Intrathecal injection; Lagrangian velocity.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable Consent for publication: All authors have approved the manuscript submission. The content of this manuscript has not been published or submitted for publication elsewhere. Competing interests: The authors declare that they have no Conflict of interest.
Figures












References
-
- Lawrence JJ, Coenen W, Sanchez AL, Pawlak G, Martinez-Bazan C, Haughton V, Lasheras JC. On the dispersion of a drug delivered intrathecally in the spinal canal. J Fluid Mech. 2019;861:679–720. 10.1017/jfm.2018.937. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

