Mitochondria-targeted antioxidant mitoquinone mitigates vitrification-induced damage in mouse ovarian tissue by maintaining mitochondrial homeostasis via the p38 MAPK pathway
- PMID: 39696534
- PMCID: PMC11657888
- DOI: 10.1186/s40001-024-02181-z
Mitochondria-targeted antioxidant mitoquinone mitigates vitrification-induced damage in mouse ovarian tissue by maintaining mitochondrial homeostasis via the p38 MAPK pathway
Abstract
Objective: Ovarian tissue cryopreservation has become a promising alternative for fertility preservation in cancer patients, allowing ovarian tissue to be stored for future autotransplantation. Oxidative stress damage occurring during the cryopreservation process may impact tissue quality and function. This study aims to investigate the protective effects and potential mechanisms of Mitoquinone (MitoQ), a mitochondria-targeted derivative of the antioxidant ubiquinone, during the vitrification of ovarian tissue in mice.
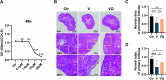
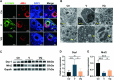
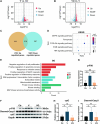
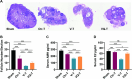
Methods: KGN cells were treated with various concentrations (0.1, 1, 10, and 50 μM) of MitoQ to determine the optimal concentration. Female ICR mice were divided into three groups: control, conventional vitrification, and MitoQ-supplemented vitrification. Ovarian samples were cryopreserved, thawed, and assessed for tissue morphology using Hematoxylin and Eosin (H&E) staining, and mitochondrial changes using immunofluorescence, transmission electron microscopy, and Western blot analysis. RNA sequencing (RNA-seq) was employed to explore potential protective mechanisms. Autotransplantation experiments were conducted, and the long-term effects of MitoQ on ovarian function were evaluated by counting follicle numbers through H&E staining and measuring serum estradiol and AMH levels using ELISA.
Results: MitoQ at 1 μM was found to be the optimal concentration for maintaining follicular morphology after vitrification. It effectively reduced mitochondrial oxidative damage, preserved mitochondrial morphology, and regulated the expression of mitochondrial dynamics proteins (Drp1 and Mfn2). RNA-seq and Western blot analyses revealed that MitoQ inhibited the p38 MAPK pathway, thereby reducing apoptosis. Additionally, autotransplantation experiments showed that MitoQ treatment significantly increased follicle counts, estradiol (E2), and anti-Müllerian hormone (AMH) levels compared to conventional vitrification.
Conclusions: MitoQ effectively mitigates vitrification-induced oxidative damage, maintains mitochondrial homeostasis, and preserves both follicular reserve and endocrine function. These findings suggest that MitoQ is a valuable adjunct in ovarian tissue cryopreservation and could significantly improve fertility preservation outcomes for cancer patients.
Keywords: Fertility preservation; Mitochondrial homeostasis; Mitoquinone; Ovarian tissue cryopreservation; Oxidative stress; p38 MAPK pathway.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committee of the Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital (Approval No. K2023097). All procedures followed the guidelines of the institution’s Animal Care and Use Committee and were in accordance with international standards for the ethical treatment of animals. Competing interests: The authors declare no competing interests.
Figures

References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. - PubMed
-
- Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53(4):727–39. - PubMed
-
- Stroud JS, Mutch D, Rader J, Powell M, Thaker PH, Grigsby PW. Effects of cancer treatment on ovarian function. Fertil Steril. 2009;92(2):417–27. - PubMed
-
- Spath MA, Braat DDM. Iatrogenic and non-iatrogenic causes of female fertility loss that may indicate fertility preservation. Acta Obstet Gynecol Scand. 2019;98(5):559–62. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

