Osteoblastic ferroptosis inhibition by small-molecule promoting GPX4 activation for peri-prosthetic osteolysis therapy
- PMID: 39696565
- PMCID: PMC11658433
- DOI: 10.1186/s12951-024-03049-4
Osteoblastic ferroptosis inhibition by small-molecule promoting GPX4 activation for peri-prosthetic osteolysis therapy
Abstract
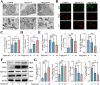
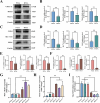
Peri-prosthesis osteolysis (PPO) represents the most severe complication of total joint arthroplasty (TJA) surgery and imposes the primary cause of prosthesis failure and subsequent revision surgery. Antiresorptive therapies are usually prescribed to treat PPO, especially for elderly people. Nevertheless, the efficacy of anti-osteoporotic medications remains constrained. Recent therapeutic strategies to promote periprosthetic osseointegration by restoring osteoblast function are considered more effective approaches. However, the precise mechanism underlying the inhibition of osteogenesis triggered by wear particles remains enigmatic. Herein, we demonstrate that wear particles inhibit osteoblast function by inducing ferroptosis to sabotage extracellular mineralization and arouse periprosthetic osteolysis. The suppression of ferroptosis could significantly rescue osteogenesis thus alleviating PPO. Furthermore, Glutathione Peroxidase 4 (GPX4) has been identified as a key target in regulating osteoblastic ferroptosis. By utilizing virtual screening techniques, we have successfully conducted a comprehensive screening of a natural compound known as Urolithin A (UA), which exhibits remarkable inhibition of osteoblastic ferroptosis while simultaneously promoting the process of osteogenesis through its precise targeting mechanism on GPX4. Meanwhile, UA improves the osteolytic conditions significantly in vivo even when the adjunction of titanium (Ti) nanoparticles. This strategy has great potential in treating peri-prosthesis osteolysis and potentially broadens the scope of clinical therapy.
Keywords: Ferroptosis; GPX4; Osteoblastogenesis; Peri-prosthesis osteolysis; Titanium nanoparticles; Urolithin A.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Clinical samples were collected with permission of the Ethics Committee of the First Affiliated Hospital of Soochow University. All animal experiments were approved by the Ethics Committee of Soochow University. Consent for publication: All authors agree to be published. Competing interests: The authors declare no competing interests.
Figures








References
MeSH terms
Substances
Grants and funding
- 82072498/National Natural Science Foundation of China
- 82072425, 82272157/National Natural Science Foundation of China
- BK2021650/Natural Science Foundation of Jiangsu Province
- ZD2022014/Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Medical Research Project
- LCZX202003/Special Project of Diagnosis; Treatment Technology for Key Clinical Diseases in Suzhou
LinkOut - more resources
Full Text Sources

