Modified vaginal lactobacilli expressing fluorescent and luminescent proteins for more effective monitoring of their release from nanofibers, safety and cell adhesion
- PMID: 39696572
- PMCID: PMC11657421
- DOI: 10.1186/s12934-024-02612-w
Modified vaginal lactobacilli expressing fluorescent and luminescent proteins for more effective monitoring of their release from nanofibers, safety and cell adhesion
Abstract
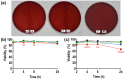
Electrospun nanofibers offer a highly promising platform for the delivery of vaginal lactobacilli, providing an innovative approach to preventing and treating vaginal infections. To advance the application of nanofibers for the delivery of lactobacilli, tools for studying their safety and efficacy in vitro need to be established. In this study, fluorescent (mCherry and GFP) and luminescent (NanoLuc luciferase) proteins were expressed in three vaginal lactobacilli (Lactobacillus crispatus, Lactobacillus gasseri and Lactobacillus jensenii) and a control Lactiplantibacillus plantarum with the aim to use this technology for close tracking of lactobacilli release from nanofibers and their adhesion on epithelial cells. The recombinant proteins influenced the growth of the bacteria, but not their ability to produce hydrogen peroxide. Survival of lactobacilli in nanofibers immediately after electrospinning varied among species. Bacteria retained fluorescence upon incorporation into PEO nanofibers, which was vital for evaluation of their rapid release. In addition, fluorescent labelling facilitated efficient tracking of bacterial adhesion to Caco-2 epithelial cells, while luminescence provided important quantitative insights into bacterial attachment, which varied from 0.5 to 50% depending on the species. The four lactobacilli in dispersion or in nanofibers were not detrimental for the viability of Caco-2 cells, and did not demonstrate hemolytic activity highlighting the safety profiles of both bacteria and PEO nanofibers. To summarize, this study contributes to the development of a promising delivery system, tailored for local administration of safe vaginal lactobacilli.
Keywords: Bioluminescence; Caco-2 cells; Electrospinning; Fluorescent proteins; NanoLuc luciferase; Nanofibers; Vaginal lactobacilli.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: The manuscript is the original work of the authors who mutually agreed on submitting the manuscript. Competing interests: The authors declare no competing interests.
Figures
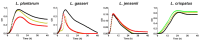







Similar articles
-
Engineering of Vaginal Lactobacilli to Express Fluorescent Proteins Enables the Analysis of Their Mixture in Nanofibers.Int J Mol Sci. 2021 Dec 20;22(24):13631. doi: 10.3390/ijms222413631. Int J Mol Sci. 2021. PMID: 34948426 Free PMC article.
-
Influence of Excipient Composition on Survival of Vaginal Lactobacilli in Electrospun Nanofibers.Pharmaceutics. 2022 May 28;14(6):1155. doi: 10.3390/pharmaceutics14061155. Pharmaceutics. 2022. PMID: 35745728 Free PMC article.
-
Putative Adhesion Factors in Vaginal Lactobacillus gasseri DSM 14869: Functional Characterization.Appl Environ Microbiol. 2019 Sep 17;85(19):e00800-19. doi: 10.1128/AEM.00800-19. Print 2019 Oct 1. Appl Environ Microbiol. 2019. PMID: 31420338 Free PMC article.
-
Vaginal microbiome.Ceska Gynekol. 2018 Winter;83(5):371-379. Ceska Gynekol. 2018. PMID: 30848142 Review. English.
-
Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health.Microb Cell Fact. 2020 Nov 7;19(1):203. doi: 10.1186/s12934-020-01464-4. Microb Cell Fact. 2020. PMID: 33160356 Free PMC article. Review.
Cited by
-
In Vitro Assessment of Biological and Functional Properties of Potential Probiotic Strains Isolated from Commercial and Dairy Sources.Microorganisms. 2025 Apr 24;13(5):970. doi: 10.3390/microorganisms13050970. Microorganisms. 2025. PMID: 40431142 Free PMC article.
-
In Vitro Evaluation of the Probiotic Properties and Whole Genome Sequencing of Lacticaseibacillus rhamnosus J3205 Isolated from Home-Made Fermented Sauce.Microorganisms. 2025 Jul 11;13(7):1643. doi: 10.3390/microorganisms13071643. Microorganisms. 2025. PMID: 40732153 Free PMC article.
-
Probiotics in Nanotechnology-Driven Wound Healing: From Mechanistic Insight to Clinical Promise.Pharmaceutics. 2025 Jun 21;17(7):805. doi: 10.3390/pharmaceutics17070805. Pharmaceutics. 2025. PMID: 40733015 Free PMC article. Review.
-
Evaluation of probiotic potential, safety assessment and whole genome sequencing of Lactiplantibacillus plantarum strain MOVIN isolated from toddy sample.Front Microbiol. 2025 Jul 10;16:1625659. doi: 10.3389/fmicb.2025.1625659. eCollection 2025. Front Microbiol. 2025. PMID: 40708923 Free PMC article.
-
Aligned Electrospun PCL/PLA Nanofibers Containing Green-Synthesized CeO2 Nanoparticles for Enhanced Wound Healing.Int J Mol Sci. 2025 Jun 25;26(13):6087. doi: 10.3390/ijms26136087. Int J Mol Sci. 2025. PMID: 40649866 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

