Sparstolonin B potentiates the antitumor activity of nanovesicle-loaded drugs by suppressing the phagocytosis of macrophages in vivo
- PMID: 39696573
- PMCID: PMC11657701
- DOI: 10.1186/s12951-024-03001-6
Sparstolonin B potentiates the antitumor activity of nanovesicle-loaded drugs by suppressing the phagocytosis of macrophages in vivo
Abstract
Background: Extracellular vesicles (EVs) and extruded nanovesicles (ENVs) are promising nanovesicles (NVs) for drug delivery. However, the application of these NVs is strongly hindered by their short half-life in the circulation. Macrophages (Mφs) in the liver and spleen contribute to the rapid depletion of NVs, but the underlying mechanism is unclear.
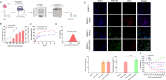
Methods: By collecting the supernatant of PANC-1 cells and squeezing PANC-1 cells, EVs and ENVs derived from PANC-1 cells were prepared via ultracentrifugation. NVs were subsequently identified via western blot, particle size measurement, and electron microscopy. The distribution of NVs in mouse bodies was observed with a live animal imaging system. Liver Mφs were extracted and isolated after NVs were administered, and transcriptome profiling was applied to determine differentially expressed genes (DEGs). siRNAs targeting interested genes were designed and synthesized. In vitro experiments, Mφs were transfected with siRNA or treated with the corresponding inhibitor, after which NV uptake was recorded. Doxorubicin (DOX) was encapsulated in ENVs using an ultrasound method. PANC-1 cell-derived tumors were established in nude mice in vivo, inhibitor pretreatment or no treatment was administered before intravenous injection of ENVs-DOX, and the therapeutic efficacy of ENVs-DOX was evaluated.
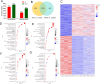
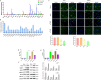
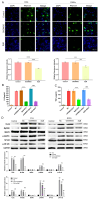
Results: NVs derived from PANC-1 cells were first prepared and identified. After intravenous injection, most NVs were engulfed by Mφs in the liver and spleen. Seven genes of interest were selected via transcriptome sequencing and validated via RT‒PCR. These results confirmed that the TLR2 signaling pathway is responsible for phagocytosis. siTLR2 and its inhibitor sparstolonin B (SpB) significantly inhibited the internalization of NVs by Mφs and downregulated the activity of the TLR2 pathway. The accumulation of ENVs-DOX in the liver was inhibited in vivo by pretreatment with SpB 40 min before intravenous injection, ultimately delaying tumor progression.
Conclusion: The TLR2 pathway plays a crucial role in the sequestration of NVs by Mφs. A novel antiphagocytic strategy in which pretreatment of mice with SpB inhibits the clearance of NVs and prolongs their half-life in vivo, thereby improving delivery efficiency, was identified.
Keywords: Drug delivery; Macrophages; Nanovesicles; Phagocytosis; Toll-like receptor 2.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experimental designs and protocols involving animals were approved by the Laboratory Animal Welfare and Ethics Committee of Renmin Hospital of Wuhan University. All procedures were conducted in accordance with the Declaration of Helsinki and standard guidelines. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Li J. A comprehensive review on the composition, biogenesis, purification, and multifunctional role of exosome as delivery vehicles for cancer therapy. Published online 2023. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

