Targeting cellular senescence in kidney diseases and aging: A focus on mesenchymal stem cells and their paracrine factors
- PMID: 39696575
- PMCID: PMC11657437
- DOI: 10.1186/s12964-024-01968-1
Targeting cellular senescence in kidney diseases and aging: A focus on mesenchymal stem cells and their paracrine factors
Abstract
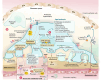
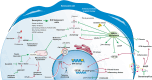
Cellular senescence is a phenomenon distinguished by the halting of cellular division, typically triggered by DNA injury or numerous stress-inducing factors. Cellular senescence is implicated in various pathological and physiological processes and is a hallmark of aging. The presence of accumulated senescent cells, whether transiently (acute senescence) or persistently (chronic senescence) plays a dual role in various conditions such as natural kidney aging and different kidney disorders. Elevations in senescent cells and senescence-associated secretory phenotype (SASP) levels correlate with decreased kidney function, kidney ailments, and age-related conditions. Strategies involving senotherapeutic agents like senolytics, senomorphics, and senoinflammation have been devised to specifically target senescent cells. Mesenchymal stem cells (MSCs) and their secreted factors may also offer alternative approaches for anti-senescence interventions. The MSC-derived secretome compromises significant therapeutic benefits in kidney diseases by facilitating tissue repair via anti-inflammatory, anti-fibrosis, anti-apoptotic, and pro-angiogenesis effects, thereby improving kidney function and mitigating disease progression. Moreover, by promoting the clearance of senescent cells or modulating their secretory profiles, MSCs could potentially reverse some age-related declines in kidney function.This review article intends to shed light on the present discoveries concerning the role of cellular senescence in kidney aging and diseases. Furthermore, it outlines the role of senotherapeutics utilized to alleviate kidney damage and aging. It also highlights the possible impact of MSCs secretome on mitigating kidney injury and prolonging lifespan across various models of kidney diseases as a novel senotherapy.
Keywords: Acute kidney injury; Cellular senescence; Chronic kidney disease; Kidney aging; Mesenchymal stem cells; Secretome of MSCs.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by Tabriz University of Medical Sciences, Tabriz, Iran (Ethical code: IR.TBZMED.REC.1402.472). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

