Regulation of dynamic spatiotemporal inflammation by nanomaterials in spinal cord injury
- PMID: 39696584
- PMCID: PMC11657436
- DOI: 10.1186/s12951-024-03037-8
Regulation of dynamic spatiotemporal inflammation by nanomaterials in spinal cord injury
Abstract
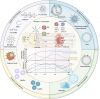
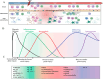
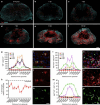
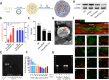
Spinal cord injury (SCI) is a common clinical condition of the central nervous system that can lead to sensory and motor impairment below the injury level or permanent loss of function in severe cases. Dynamic spatiotemporal neuroinflammation is vital to neurological recovery, which is collectively constituted by the dynamic changes in a series of inflammatory cells, including microglia, neutrophils, and astrocytes, among others. Immunomodulatory nanomaterials can readily improve the therapeutic effects and simultaneously overcome various drawbacks associated with treatment, such as the off-target side effects and loss of bioactivity of immune agents during circulation. In this review, we discuss the role of dynamic spatiotemporal inflammation in secondary injuries after SCI, elaborate on the mechanism of action and effect of existing nanomaterials in treating SCI, and summarize the mechanism(s) whereby they regulate inflammation. Finally, the challenges and prospects associated with using nanotechnology to modulate immunotherapy are discussed to provide new insights for future treatment. Deciphering the intricate spatiotemporal mechanisms of neuroinflammation in SCI requires further in-depth studies. Therefore, SCI continues to represent a formidable challenge.
Keywords: Astrocyte; Inflammation; Microglia; Nanomaterials; Spatiotemporal dynamic; Spinal cord injury.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- Tamburin S, Filippetti M, Mantovani E, Smania N, Picelli A. Spasticity following brain and spinal cord injury: assessment and treatment. Curr Opin Neurol. 2022;35(6):728–40. - PubMed
Publication types
MeSH terms
Grants and funding
- 82401619/National Natural Science Foundation of China
- 51803072/National Natural Science Foundation of China
- 82271411/National Natural Science Foundation of China
- 2022qnpy11/Youth Support Programmed Project of China Japan Union Hospital of Jilin University
- 2022SCZ25/Department of Finance of Jilin Province
LinkOut - more resources
Full Text Sources
Medical

