Impact of noradrenergic inhibition on neuroinflammation and pathophysiology in mouse models of Alzheimer's disease
- PMID: 39696597
- PMCID: PMC11657531
- DOI: 10.1186/s12974-024-03306-1
Impact of noradrenergic inhibition on neuroinflammation and pathophysiology in mouse models of Alzheimer's disease
Abstract
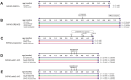
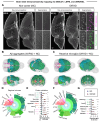
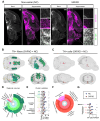
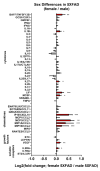
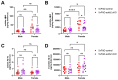
Norepinephrine (NE) modulates cognitive function, arousal, attention, and responses to novelty and stress, and it also regulates neuroinflammation. We previously demonstrated behavioral and immunomodulatory effects of beta-adrenergic pharmacology in mouse models of Alzheimer's disease (AD). The current studies were designed to block noradrenergic signaling in 5XFAD mice through (1) chemogenetic inhibition of the locus coeruleus (LC), (2) pharmacologic blocking of β-adrenergic receptors, and (3) conditional deletion of β1- or β2-adrenergic receptors (adrb1 or adrb2) in microglia.First, brain-wide AD pathology was mapped in 3D by imaging immunolabeled, cleared 5XFAD brains to assess the overlap between amyloid beta (Aβ) pathology, reactive microglia, and the loss of tyrosine hydroxylase (TH) expression in the catecholaminergic system. To examine the effects of inhibiting the LC NE system in the 5XFAD model, inhibitory (Gi) DREADD receptors were expressed specifically in LC NE neurons. LC NE neurons were chronically inhibited through the subcutaneous pump administration of the DREADD agonist clozapine-N-oxide (CNO). Plasma and brains were collected for assessment of neuroinflammation and pathology. A separate cohort of 5XFAD mice was chronically dosed with the beta-adrenergic antagonist propranolol or vehicle and evaluated for behavior, as well as post-mortem neuroinflammation and pathology. Finally, we used 5XFAD mice with conditional deletion of either adrb1 or adrb2 in microglia to assess neuroinflammation and pathology mediated by β-adrenergic signaling.Using iDISCO+, light sheet fluorescence microscopy, and novel analyses, we detected widespread microgliosis and Aβ pathology, along with modest TH downregulation in fibers across multiple brain regions, in contrast to the spatially limited TH downregulation observed in neurons. Both chemogenetic inhibition of LC adrenergic signaling and pharmacological inhibition of beta-adrenergic receptors potentiated neuroinflammation without altering Aβ pathology. Conditional deletion of adrb1 in microglia did not affect neuroinflammation. Conditional deletion of adrb2 in microglia attenuated inflammation and pathology in females but had no effect in males. Overall, these data support previous observations demonstrating the immunomodulatory effects of beta-adrenergic signaling in the pathophysiology of brain disorders and suggest that adrenergic receptors on cell types other than microglia, such as astrocytes, may mediate some of the disease-modifying effects of β-adrenergic agonists in the brain.
Keywords: Alzheimer’s Disease; Amyloid beta; Beta-adrenergic receptor; Beta-blocker; Locus coeruleus; Microglia; Neuroinflammation; Norepinephrine; iDISCO+.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: MS is a shareholder and founder of CuraSen Therapeutics, a company developing adrenergic drugs for the treatment of neurodegenerative disease. AKE is a shareholder of CuraSen Therapeutics. However, this work was initiated and carried out independently from CuraSen Therapeutics and was not designed, initiated, or funded by CuraSen Therapeutics. BDH is on the scientific advisory boards of Journey Clinical and Osmind, and is a paid consultant to Arcadia Medicine, Inc.
Figures





Update of
-
Impact of noradrenergic inhibition on neuroinflammation and pathophysiology in mouse models of Alzheimer's disease.Res Sq [Preprint]. 2024 Oct 30:rs.3.rs-5328229. doi: 10.21203/rs.3.rs-5328229/v1. Res Sq. 2024. Update in: J Neuroinflammation. 2024 Dec 18;21(1):322. doi: 10.1186/s12974-024-03306-1. PMID: 39574895 Free PMC article. Updated. Preprint.
References
-
- Matt RA, Martin RS, Evans AK, Gever JR, Vargas GA, Shamloo M et al. Locus Coeruleus and Noradrenergic Pharmacology in Neurodegenerative Disease. Handb Exp Pharmacol. 2023. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

