Altered metabolic function induced by Aβ-oligomers and PSEN1 mutations in iPSC-derived astrocytes
- PMID: 39696767
- PMCID: PMC11655965
- DOI: 10.1111/jnc.16267
Altered metabolic function induced by Aβ-oligomers and PSEN1 mutations in iPSC-derived astrocytes
Abstract
Altered energy metabolism in Alzheimer's disease (AD) is a major pathological hallmark implicated in the early stages of the disease process. Astrocytes play a central role in brain homeostasis and are implicated in multiple neurodegenerative diseases. Although numerous studies have investigated global changes in brain metabolism, redox status, gene expression and epigenetic markers in AD, the intricate interplay between different metabolic processes, particularly in astrocytes, remains poorly understood. Numerous studies have implicated amyloid-β and the amyloid-β precursor in the development and progression of AD. To determine the effects of amyloid-β peptides or the impact of amyloid-β precursor protein processing on astrocyte metabolism, we differentiated astrocytes from induced pluripotent stem cells derived from people with early onset familial AD and controls. This study demonstrates that familial AD-derived astrocytes exhibit significantly more changes in their metabolism including glucose uptake, glutamate uptake and lactate release, with increases in oxidative and glycolytic metabolism compared to acute amyloid-β exposure. In addition to changes in major metabolic pathways including glutamate, purine and arginine metabolism and the citric acid cycle, we demonstrate evidence of gliosis in familial AD astrocytes highlighting a potential pathological hallmark. This suggests that chronic alterations in metabolism may occur very early in the disease process and present significant risk factors for disease progression for patients with early onset AD. These findings may also reveal important drivers of disease in late onset dementia and highlights key targets for potential diagnostic features and therapeutic agents in the future.
Keywords: Alzheimer's; astrocytes; gliosis; inflammation; metabolism; stem cells.
© 2024 The Author(s). Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
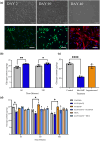



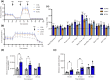


References
-
- Abdelrazig, S. , Safo, L. , Rance, G. A. , Fay, M. W. , Theodosiou, E. , Topham, P. D. , Kim, D. H. , & Fernández‐Castané, A. (2020). Metabolic characterisation of Magnetospirillum gryphiswaldense MSR‐1 using LC‐MS‐based metabolite profiling. RSC Advances, 10, 32548–32560. 10.1039/d0ra05326k - DOI - PMC - PubMed
-
- Allaman, I. , Gavillet, M. , Bélanger, M. , Laroche, T. , Viertl, D. , Lashuel, H. A. , & Magistretti, P. J. (2010). Amyloid‐beta aggregates cause alterations of astrocytic metabolic phenotype: Impact on neuronal viability. The Journal of Neuroscience, 30, 3326–3338. 10.1523/jneurosci.5098-09.2010 - DOI - PMC - PubMed
-
- Amaral, A. I. , Teixeira, A. P. , Håkonsen, B. I. , Sonnewald, U. , & Alves, P. M. (2011). A comprehensive metabolic profile of cultured astrocytes using isotopic transient metabolic flux analysis and C‐labeled glucose. Frontiers in Neuroenergetics, 3, 5. 10.3389/fnene.2011.00005 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

