Long-term weight loss and cardiorenal outcomes by baseline BMI in the VERTIS CV trial
- PMID: 39696829
- PMCID: PMC11701189
- DOI: 10.1111/dom.16050
Long-term weight loss and cardiorenal outcomes by baseline BMI in the VERTIS CV trial
Abstract
Aim: To assess weight loss and cardiorenal outcomes by baseline body mass index (BMI) in VERTIS CV.
Methods: Patients with type 2 diabetes and atherosclerotic cardiovascular (CV) disease were randomized to ertugliflozin or placebo. These post hoc analyses evaluated cardiometabolic and cardiorenal outcomes (a composite of death from CV causes or hospitalization for heart failure [HHF], CV death, HHF and an exploratory composite kidney outcome including ≥40% estimated glomerular filtration rate [eGFR] decrease) by baseline BMI, using conventional clinical categories and Cox proportional hazards models.
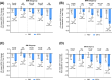
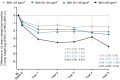
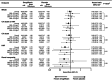
Results: In total, 8246 adults were randomized (mean age 64.4 years, diabetes duration 13.0 years, BMI 32.0 kg/m2, 61% with BMI >30 kg/m2). Absolute body weight reduction was greater with ertugliflozin versus placebo at 3 and 5 years in the overall population (p < 0.001) and across BMI subgroups. Ertugliflozin increased the proportion of participants achieving ≥5% and ≥10% body weight reduction (ertugliflozin 34.9% and 13.6%, placebo 19.4% and 4.1%; odds ratio [95% confident interval, CI], 2.21 [1.76-2.77] and 3.65 [2.39-5.57], respectively) at 5 years. No significant difference was observed in the effect of ertugliflozin on HHF across BMI subgroups (Pinteraction = 0.61). Similarly, no significant difference was observed in the effect of ertugliflozin on the kidney composite outcome across BMI subgroups (Pinteraction = 0.39). Results were similar for other CV outcomes, and safety was consistent with the known ertugliflozin profile.
Conclusion: Weight loss was observed across baseline BMI and was sustained over 5 years of follow-up. The effects of ertugliflozin on HHF and kidney composite were consistent across baseline BMI.
Keywords: body mass index; cardiovascular and kidney outcomes; ertugliflozin; metabolic outcomes; type 2 diabetes.
© 2024 Pfizer Inc. Merck Sharp & Dohme LLC and The Author(s). Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

