Development of a validated novel bead extraction method for the detection of anti-PEG antibodies in human serum
- PMID: 39696894
- PMCID: PMC11749383
- DOI: 10.1080/17576180.2024.2442198
Development of a validated novel bead extraction method for the detection of anti-PEG antibodies in human serum
Abstract
Aims: Polyethylene glycol (PEG) is used in many applications including drug development. Due to exposure to environmental products, there is a high prevalence of preexisting anti-PEG antibodies in the global human population. The presence of anti-PEG antibodies is a concern for potentially reducing the efficacy of therapeutics after administration and represents a risk of safety events after exposure to PEGylated drug products. We developed and validated a creative and sensitive method for the detection of anti-PEG antibodies in human serum to support clinical programs for PEGylated drugs.
Methods: In this method, biotin-PEG streptavidin beads were used to extract anti-PEG antibodies from human serum for analysis in an anti-PEG ELISA assay. The same serum sample was analyzed in an anti-drug antibody assay.
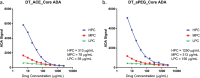
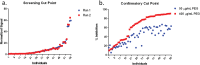
Results: The anti-PEG antibody assay was validated with a screening cut point of 1.41 normalized signal, confirmatory cut point of 32.2% inhibition, sensitivity of 7.81 ng/mL and sufficient reproducibility, selectivity, and drug tolerance in accordance with the FDA 2019 Immunogenicity guidance.
Conclusion: This method of removal of anti-PEG antibodies enables the use of a single sample to detect anti-drug and anti-PEG antibodies to support drug development programs.
Keywords: ADA; BEAD; PEG; anti-PEG antibodies; immunogenicity.
Conflict of interest statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
