Genetic variation on dolutegravir pharmacokinetics and relation to safety and efficacy outcomes: a systematic review
- PMID: 39697075
- PMCID: PMC11703464
- DOI: 10.1080/14622416.2024.2441104
Genetic variation on dolutegravir pharmacokinetics and relation to safety and efficacy outcomes: a systematic review
Abstract
Background: Dolutegravir (DTG) is an antiviral agent used for the treatment of HIV, however, there is uncertainty over the influence of genetic variation on DTG exposure, and whether it has clinical implications for the efficacy or toxicity in different populations. This systematic review aims to create an overview of the impact of pharmacogenomics (PGx) on DTG exposure, efficacy, and toxicity.
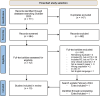
Methods: Publications up to 14 November 2023 were searched and articles were selected on the following criteria: original research articles providing data on people with HIV, data on PGx and either PK or PD or both PD and PGx.
Results: 711 records were identified, and after screening 10 articles were included. Commonly analyzed genes across the articles were UGT1A1, ABCB1, ABCG2, and NR1I2. The most reported variant associated with PD variability was in SLC22A2, with carriers at higher risk of neuropsychiatric adverse events.
Conclusions: This review concludes that while PGx testing may help explain some variability in DTG pharmacokinetics when combined with therapeutic drug monitoring (TDM), current evidence is insufficient to support its routine clinical use. The role of PGx research for DTG remains relevant, especially in specific patient populations where interindividual PK variations are still unexplained.
Keywords: Dolutegravir; HIV; antiretroviral; pharmacodynamics; pharmacogenomics; pharmacokinetics.
Conflict of interest statement
AC received research grants from ViiV Healthcare, Gilead and Merck, paid to the institution. DC has received research funding from ViiV Healthcare. DB has been a member of advisory boards of and received research grants from ViiV Healthcare. AO is a Director of Tandem Nano Ltd and co-inventor of patents relating to drug delivery. AO has been co-investigator on funding received by the University of Liverpool from ViiV Healthcare and Gilead Sciences in the past 3 years unrelated to the current paper. AO has received personal fees from Gilead Sciences, Assembly Biosciences and Shionogi in the past 3 years also unrelated to the current paper. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
References
-
- Organisation WH . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring. 2021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical