Development of a Microphysiological Cartilage-on-Chip Platform for Dynamic Biomechanical Stimulation of Three-Dimensional Encapsulated Chondrocytes in Agarose Hydrogels
- PMID: 39697087
- PMCID: PMC11656284
- DOI: 10.1002/cpz1.70079
Development of a Microphysiological Cartilage-on-Chip Platform for Dynamic Biomechanical Stimulation of Three-Dimensional Encapsulated Chondrocytes in Agarose Hydrogels
Abstract
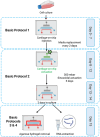
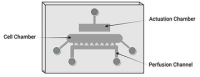
Osteoarthritis (OA) is one of the most prevalent joint diseases globally, characterized by the progressive breakdown of articular cartilage, resulting in chronic pain, stiffness, and loss of joint function. Despite its significant socioeconomic impact, therapeutic options remain limited, largely due to an incomplete understanding of the molecular mechanisms driving cartilage degradation and OA pathogenesis. Recent advances in in vitro modeling have revolutionized joint tissue research, transitioning from simplistic two-dimensional cell cultures to sophisticated three-dimensional (3D) constructs that more accurately mimic the physiological microenvironment of native cartilage. Over the last decade, organ-on-chip technologies have emerged as transformative tools in tissue engineering, offering microphysiological platforms with precise control over biomechanical and biochemical stimuli. These platforms are providing novel insights into tissue responses and disease progression and are increasingly integrated into the early stages of drug screening and development. In this article, we present a detailed experimental protocol for constructing a cartilage-on-chip system capable of delivering controlled dynamic biomechanical stimulation to 3D-encapsulated chondrocytes in an agarose hydrogel matrix. Our protocol, optimized for both bovine and human chondrocytes, begins with Basic Protocol 1, detailing the preparation and injection of cell-laden hydrogels into the microdevice. Basic Protocol 2 describes the application of dynamic mechanical loading using a calibrated pressurized pump. Finally, Basic Protocols 3 and 4 focus on the retrieval of the hydrogel and RNA extraction for downstream molecular analyses. This platform represents a critical advancement for in vitro studies of cartilage biology, enabling more precise modeling of OA pathophysiology and evaluation of experimental therapeutics. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC. Basic Protocol 1: Cartilage-on-chip injection Basic Protocol 2: Cartilage-on-chip actuation Basic Protocol 3: Cartilage-on-chip agarose hydrogel removal Basic Protocol 4: Preparation of cartilage-on-chip for RNA extraction.
Keywords: cartilage; chondrocyte; microphysiological; organ‐on‐a‐chip; osteoarthritis.
© 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.
Conflict of interest statement
Carlo Alberto Paggi and Elsa Lauwers work for chrn on‐chip biotechnology B.V. and have helped in the protocol drafting.
Figures











References
Literature Cited
-
- Buckwalter, J. A. , Mankin, H. J. , & Grodzinsky, A. J. (2005). Articular cartilage and osteoarthritis. Instructional Course Lectures, 54, 465–80. - PubMed
-
- Buckwalter, J. A. , Martin, J. A. , & Brown, T. D. (2006). Perspectives on chondrocyte mechanobiology and osteoarthritis. Biorheology, 43(3,4), 603–609. - PubMed
-
- Cross, M. , Smith, E. , Hoy, D. , Nolte, S. , Ackerman, I. , Fransen, M. , Bridgett, L. , Williams, S. , Guillemin, F. , Hill, C. L. , Laslett, L. L. , Jones, G. , Cicuttini, F. , Osborne, R. , Vos, T. , Buchbinder, R. , Woolf, A. , & March, L. (2014). The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Annals of the Rheumatic Diseases, 73(7), 1323–1330. 10.1136/annrheumdis-2013-204763 - DOI - PubMed
Internet Resources
-
- chiron's website.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

