Skeletal Phenotyping of Period-1-Deficient Melatonin-Proficient Mice
- PMID: 39697088
- PMCID: PMC11656283
- DOI: 10.1111/jpi.70020
Skeletal Phenotyping of Period-1-Deficient Melatonin-Proficient Mice
Abstract
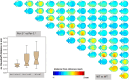
In mice, variability in adult bone size and density has been observed among common inbred strains. Also, in the group of genes regulating circadian rhythmicity in mice, so called clock genes, changes in body size and skeletal parameters have been noted in knockout mice. Here, we studied the size and density of prominent bones of the axial and appendicular skeleton of clock gene Period-1-deficient (Per1-/-) mice by means of microcomputed tomography. Our data show shorter spinal length, smaller and less dense femora and tibiae, but no significant changes in the shape of the skull and the length of the head. Together with the significantly lower total body weight of Per1-/- mice, we conclude that Per1-deficiency in a melatonin-proficient mouse strain is associated with an altered body phenotype with smaller appendicular (hind limb) bone size, shorter spine length and lower total body weight while normal head length and brain weight. The observed changes suggest an involvement of secondary bone mineralisation with impact on long bones, but lesser impact on those of the skull. Evidence and overall physiological implications of these findings are discussed.
Keywords: bone density; clock genes; microcomputed tomography; period‐1; body size regulation; ossification.
© 2024 The Author(s). Journal of Pineal Research published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



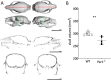

References
-
- Albrecht U., “Timing to Perfection: The Biology of Central and Peripheral Circadian Clocks,” Neuron 74 (2012): 246–260. - PubMed
-
- Albrecht U., Sun Z. S., Eichele G., and Lee C. C., “A Differential Response of Two Putative Mammalian Circadian Regulators, mper1 and mper2, to Light,” Cell 91 (1997): 1055–1064. - PubMed
-
- Bab I. A., Hajbi‐Yonissi C., Gabet Y., and Müller R., Micro‐Tomographic Atlas of the Mouse Skeleton (Springer Science & Business Media, 2007).
-
- Bae K., Jin X., Maywood E. S., Hastings M. H., Reppert S. M., and Weaver D. R., “Differential Functions of mPer1, mPer2, and mPer3 in the SCN Circadian Clock,” Neuron 30 (2001): 525–536. - PubMed
-
- Bae K., Lee K., Seo Y., Lee H., Kim D., and Choi I., “Differential Effects of Two Period Genes on the Physiology and Proteomic Profiles of Mouse Anterior Tibialis Muscles,” Molecules and Cells 22 (2006): 275–284. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

