Pyruvate Abundance Confounds Aminoglycoside Killing of Multidrug-Resistant Bacteria via Glutathione Metabolism
- PMID: 39697188
- PMCID: PMC11654824
- DOI: 10.34133/research.0554
Pyruvate Abundance Confounds Aminoglycoside Killing of Multidrug-Resistant Bacteria via Glutathione Metabolism
Abstract
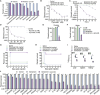
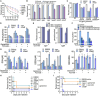
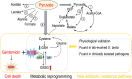
To explore whether the metabolic state reprogramming approach may be used to explore previously unknown metabolic pathways that contribute to antibiotic resistance, especially those that have been neglected in previous studies, pyruvate reprogramming was performed to reverse the resistance of multidrug-resistant Edwardsiella tarda. Surprisingly, we identified a pyruvate-regulated glutathione system that occurs by boosting glycine, serine, and threonine metabolism. Moreover, cysteine and methionine metabolism played a key role in this reversal. This process involved pyruvate-depressed glutathione and pyruvate-promoted glutathione oxidation, which was attributed to the elevated glutathione peroxidase and depressed glutathione reductase that was inhibited by glycine. This regulation inhibited reactive oxygen species (ROS) degradation and thereby elevated ROS to eliminate E. tarda. Loss of metB, gpx, and gor of the metabolic pathways increased and decreased resistance, respectively, both in vitro and in vivo, thereby supporting the hypothesis of a pyruvate-cysteine-glutathione system/glycine-ROS metabolic pathway. The role of this metabolic pathway in drug resistance and reprogramming reversal was demonstrated in laboratory-evolved gentamicin-resistant E. tarda and other clinically isolated multidrug- and carbapenem-resistant pathogens. Thus, we reveal a less studied antibiotic resistance metabolic pathway along with the mechanisms involved in its reversal.
Copyright © 2024 Jiao Xiang et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures






Similar articles
-
Enhanced Biosynthesis of Fatty Acids Is Associated with the Acquisition of Ciprofloxacin Resistance in Edwardsiella tarda.mSystems. 2021 Aug 31;6(4):e0069421. doi: 10.1128/mSystems.00694-21. Epub 2021 Aug 24. mSystems. 2021. PMID: 34427511 Free PMC article.
-
Reactive Oxygen Species-Related Ceftazidime Resistance Is Caused by the Pyruvate Cycle Perturbation and Reverted by Fe3 + in Edwardsiella tarda.Front Microbiol. 2021 Apr 28;12:654783. doi: 10.3389/fmicb.2021.654783. eCollection 2021. Front Microbiol. 2021. PMID: 33995314 Free PMC article.
-
Identification and efficacy of glycine, serine and threonine metabolism in potentiating kanamycin-mediated killing of Edwardsiella piscicida.J Proteomics. 2018 Jul 15;183:34-44. doi: 10.1016/j.jprot.2018.05.006. Epub 2018 May 9. J Proteomics. 2018. PMID: 29753025
-
Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism.Free Radic Biol Med. 2013 Dec;65:1538-1547. doi: 10.1016/j.freeradbiomed.2013.07.016. Epub 2013 Jul 18. Free Radic Biol Med. 2013. PMID: 23872396 Review.
-
Anaerobic bacterial metabolism in the ancient eukaryote Giardia duodenalis.Int J Parasitol. 1998 Jan;28(1):149-64. doi: 10.1016/s0020-7519(97)00172-0. Int J Parasitol. 1998. PMID: 9504342 Review.
Cited by
-
Metabolomic Profiling and Anti-Helicobacter pylori Activity of Caulerpa lentillifera (Sea Grape) Extract.Mar Drugs. 2025 Jul 7;23(7):282. doi: 10.3390/md23070282. Mar Drugs. 2025. PMID: 40710507 Free PMC article.
-
Metabolic state-driven nutrient-based approach to combat bacterial antibiotic resistance.NPJ Antimicrob Resist. 2025 Apr 4;3(1):24. doi: 10.1038/s44259-025-00092-5. NPJ Antimicrob Resist. 2025. PMID: 40185857 Free PMC article. Review.
-
Glutamine potentiates cefoperazone-sulbactam activity against Pseudomonas aeruginosa by increasing membrane permeability and cellular uptake.Front Microbiol. 2025 Jul 3;16:1631646. doi: 10.3389/fmicb.2025.1631646. eCollection 2025. Front Microbiol. 2025. PMID: 40678050 Free PMC article.
-
Metabolic reprogramming enhances the susceptibility of multidrug- and carbapenem-resistant bacteria to antibiotics.Nat Microbiol. 2025 Aug 11. doi: 10.1038/s41564-025-02083-8. Online ahead of print. Nat Microbiol. 2025. PMID: 40790107
References
-
- Peng B, Li H, Peng XX. Proteomics approach to understand bacterial antibiotic resistance strategies. Expert Rev Proteomics. 2019;16:829–839. - PubMed
-
- Luepke KH, Mohr JF. The antibiotic pipeline: Reviving research and development and speeding drugs to market. Expert Rev Anti-Infect Ther. 2017;15(5):425–433. - PubMed
LinkOut - more resources
Full Text Sources

