Safety and efficacy of 48-week pegylated interferon- α-2b therapy in patients with hepatitis B virus-related compensated liver cirrhosis: a pilot observational study
- PMID: 39697201
- PMCID: PMC11652151
- DOI: 10.3389/fmed.2024.1489671
Safety and efficacy of 48-week pegylated interferon- α-2b therapy in patients with hepatitis B virus-related compensated liver cirrhosis: a pilot observational study
Abstract
Background: Pegylated interferon-α (PEG-IFN-α) therapy could decrease hepatitis B surface antigen (HBsAg) and improve long-term prognosis of hepatitis B virus (HBV) infection. However, studies on safety and efficacy of PEG-IFN-α for patients with HBV-related cirrhosis are limited.
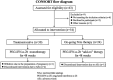
Methods: This was a single-center study. Fifty-four patients with HBV-related compensated cirrhosis were enrolled. All patients received subcutaneous injection of PEG-IFN-α-2b 180 μg per week for 48 weeks. The monotherapy of PEG-IFN-α-2b was used for treatment-naïve patients, while addition of PEG-IFN-α-2b to on-going nucleos(t)ide analogs (NAs) was used for NAs-experienced patients. Clinical symptoms, laboratory tests, examination indicators, and adverse events were collected at each observational time point.
Results: Forty-two patients achieved undetectable serum HBV DNA at 48 weeks post-therapy. HBsAg level was significantly reduced at 48 weeks post-therapy (227.2 IU/mL vs. 1,668 IU/mL; p < 0.001), especially in NAs-experienced patients (161.0 IU/mL vs. 1,207 IU/mL; p = 0.005). Three patients achieved HBsAg loss, and two of them obtained HBsAg seroconversion. There were no significant differences in liver stiffness measurement, thickness and length of spleen, or diameter of portal vein between baseline and 48 weeks post-therapy (p > 0.05). The aminotransferase levels were increased, while white blood cells, neutrophils, and platelets counts were decreased during PEG-IFN-α-2b therapy (p < 0.05), especially in treatment-naïve patients. Three patients discontinued PEG-IFN-α-2b therapy due to severe adverse events. No patients suffered with virological breakthrough or progressed to end-stage liver diseases during observational period.
Conclusion: A finite course of PEG-IFN-α-2b therapy was well-tolerated, and reduced HBsAg level without accelerating disease progression in patients with HBV-related compensated cirrhosis.
Clinical trial registration: This trial is a part of ZhuFeng Project (ClinicalTrials.gov, identifier NCT04035837).
Keywords: antiviral therapy; clinical cure; hepatitis B virus; liver cirrhosis; pegylated interferon-α.
Copyright © 2024 Wang, Wang, Zhou, Shi, Hua and Feng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Associated data
LinkOut - more resources
Full Text Sources
Medical

