Targeting the SMURF2-HIF1α axis: a new frontier in cancer therapy
- PMID: 39697237
- PMCID: PMC11652374
- DOI: 10.3389/fonc.2024.1484515
Targeting the SMURF2-HIF1α axis: a new frontier in cancer therapy
Abstract
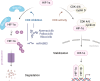
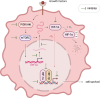
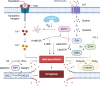
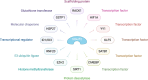
The SMAD-specific E3 ubiquitin protein ligase 2 (SMURF2) has emerged as a critical regulator in cancer biology, modulating the stability of Hypoxia-Inducible Factor 1-alpha (HIF1α) and influencing a network of hypoxia-driven pathways within the tumor microenvironment (TME). SMURF2 targets HIF1α for ubiquitination and subsequent proteasomal degradation, disrupting hypoxic responses that promote cancer cell survival, metabolic reprogramming, angiogenesis, and resistance to therapy. Beyond its role in HIF1α regulation, SMURF2 exerts extensive control over cellular processes central to tumor progression, including chromatin remodeling, DNA damage repair, ferroptosis, and cellular stress responses. Notably, SMURF2's ability to promote ferroptotic cell death through GSTP1 degradation offers an alternative pathway to overcome apoptosis resistance, expanding therapeutic options for refractory cancers. This review delves into the multifaceted interactions between SMURF2 and HIF1α, emphasizing how their interplay impacts metabolic adaptations like the Warburg effect, immune evasion, and therapeutic resistance. We discuss SMURF2's dual functionality as both a tumor suppressor and, in certain contexts, an oncogenic factor, underscoring its potential as a highly versatile therapeutic target. Furthermore, modulating the SMURF2-HIF1α axis presents an innovative approach to destabilize hypoxia-dependent pathways, sensitizing tumors to chemotherapy, radiotherapy, and immune-based treatments. However, the complexity of SMURF2's interactions necessitate a thorough assessment of potential off-target effects and challenges in specificity, which must be addressed to optimize its clinical application. This review concludes by proposing future directions for research into the SMURF2-HIF1α pathway, aiming to refine targeted strategies that exploit this axis and address the adaptive mechanisms of aggressive tumors, ultimately advancing the landscape of precision oncology.
Keywords: HIF1α; SMURF2; angiogenesis; cancer therapy; ferroptosis; hypoxia; metabolic reprogramming; tumor microenvironment.
Copyright © 2024 Youssef, Zhao, Purcell, Olson and El-Deiry.
Conflict of interest statement
EY is employed by SMURF-Therapeutics, Inc. GO is employed by Provid Pharmaceuticals Inc. WE-D is the Scientific Founder and Shareholder of Oncoceutics, Inc. acquired by Chimerix, p53-Therapeutics, Inc., and SMURF-Therapeutics, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

