Functionalization of 3D printed poly(lactic acid)/graphene oxide/β-tricalcium phosphate (PLA/GO/TCP) scaffolds for bone tissue regeneration application
- PMID: 39697249
- PMCID: PMC11651288
- DOI: 10.1039/d4ra05889e
Functionalization of 3D printed poly(lactic acid)/graphene oxide/β-tricalcium phosphate (PLA/GO/TCP) scaffolds for bone tissue regeneration application
Abstract
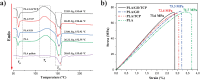
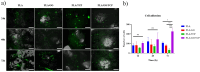
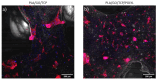
The challenge of bone tissue regeneration implies the use of new advanced technologies for the manufacture of polymeric matrices, with 3D printing technology being a suitable option for tissue engineering due to its low processing cost, its simple operation and the wide use of biomaterials in biomedicine. Among the biopolymers used to obtain porous scaffolds, poly(lactic acid) (PLA) stands out due its mechanical and biodegradability properties, although its low bioactivity to promote bone regeneration is a great challenge. In this research, a 3D scaffold based on PLA reinforced with bioceramics such as graphene oxide (GO) and β-tricalcium phosphate (TCP) was designed and characterized by FTIR, XRD, DSC, SEM and mechanical tests. The in vitro biocompatibility, viability, and cell proliferation of the poly-l-lysine (POLYL) functionalized scaffold were investigated using Wharton Jelly mesenchymal stem cells (hWJ-MSCs) and confirmed by XPS. The incorporation of GO/TCP bioceramics into the PLA polymer matrix increased the mechanical strength and provided a thermal barrier during the fusion treatments that the polymeric material undergoes during its manufacturing. The results show that the functionalization of the scaffold with POLYL allows improving the cell adhesion, proliferation and differentiation of hWJ-MSCs. The resulting scaffold PLA/GO/TCP/POLYL exhibits enhanced structural integrity and osteogenic cues, rendering it a promising candidate for biomedical applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have no conflicts of interest to declare with respect to the research, authorship and publication of this article.
Figures







Similar articles
-
Effect of Silicon Dioxide and Magnesium Oxide on the Printability, Degradability, Mechanical Strength and Bioactivity of 3D Printed Poly (Lactic Acid)-Tricalcium Phosphate Composite Scaffolds.Tissue Eng Regen Med. 2024 Feb;21(2):223-242. doi: 10.1007/s13770-023-00584-3. Epub 2023 Oct 19. Tissue Eng Regen Med. 2024. PMID: 37856070 Free PMC article.
-
Fabrication and properties of PLA/β-TCP scaffolds using liquid crystal display (LCD) photocuring 3D printing for bone tissue engineering.Front Bioeng Biotechnol. 2024 Feb 19;12:1273541. doi: 10.3389/fbioe.2024.1273541. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38440328 Free PMC article.
-
In Vitro Mechanical and Biological Properties of 3D Printed Polymer Composite and β-Tricalcium Phosphate Scaffold on Human Dental Pulp Stem Cells.Materials (Basel). 2020 Jul 8;13(14):3057. doi: 10.3390/ma13143057. Materials (Basel). 2020. PMID: 32650530 Free PMC article.
-
Applications of 3D printed bone tissue engineering scaffolds in the stem cell field.Regen Ther. 2021 Feb 5;16:63-72. doi: 10.1016/j.reth.2021.01.007. eCollection 2021 Mar. Regen Ther. 2021. PMID: 33598507 Free PMC article. Review.
-
Synthetic Biodegradable Aliphatic Polyester Nanocomposites Reinforced with Nanohydroxyapatite and/or Graphene Oxide for Bone Tissue Engineering Applications.Nanomaterials (Basel). 2019 Apr 10;9(4):590. doi: 10.3390/nano9040590. Nanomaterials (Basel). 2019. PMID: 30974820 Free PMC article. Review.
References
-
- N. I. o. B. I. a. B. (NIBIB), Ingeniería de Tejidos y Medicina Regenerativa, https://www.nibib.nih.gov/espanol/temas-cientificos/ingenier%C3%ADa-de-t..., (accessed March, 2024)
-
- Li X. Wang L. Fan Y. Feng Q. Cui F. Z. Watari F. J. Biomed. Mater. Res. A. 2013;101:2424–2435. - PubMed
-
- Salamanca E. Tsao T.-C. Hseuh H.-W. Wu Y.-F. Choy C.-S. Lin C.-K. Pan Y.-H. Teng N.-C. Huang M.-C. Lin S.-M. Front. Mater. 2021;8:683706.
LinkOut - more resources
Full Text Sources

