Integrating spore trapping technology with loop-mediated isothermal amplification assay for surveillance and sustainable management of rice false smut disease
- PMID: 39697657
- PMCID: PMC11652660
- DOI: 10.3389/fmicb.2024.1485275
Integrating spore trapping technology with loop-mediated isothermal amplification assay for surveillance and sustainable management of rice false smut disease
Abstract
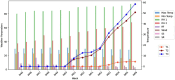
Rice (Oryza sativa L.) is a vital crop feeding more than half of the world's population, with production occurring predominantly in Asian countries. However, rice cultivation faces challenges from various fronts, including biotic stresses intensified by climate change. False smut, caused by Ustilaginoidea virens, has emerged as a significant threat to rice production globally. The application of curative fungicides after symptom appearance has limited scope in managing this disease since the infection process usually starts during the early flowering stage of rice crops. This study investigates the utilization of spore-trapping technology coupled with Loop-Mediated Isothermal Amplification (LAMP) assay for monitoring airborne U. virens inocula in rice fields. For early detection and quantification of U. virens, sampling rods coated with silicone grease were deployed to collect airborne spores, and DNA extraction was performed using a modified method. Both PCR and LAMP assays were employed for detection, with LAMP offering advantages of rapidity, sensitivity, and simplicity. The study demonstrated the superior sensitivity of LAMP compared to PCR, detecting U. virens DNA at concentrations as low as 100 femtograms. Continuous monitoring of U. virens inoculum using spore trapping revealed the spatio-temporal dynamics of U. virens dispersal, providing valuable insights for disease management. Implementing a fungicidal application schedule based on airborne inoculum detection led to significant reductions in both false smut incidence and severity and improved crop yield. The meteorological parameters including minimum temperature, relative humidity in the morning and evening, sunshine, and solar radiation were found to be correlated with disease incidence. Multi-operator validation confirmed the robustness and specificity of the LAMP assay. Overall, this integrated approach offers a proactive strategy for monitoring and managing false smut disease, enhancing sustainable rice production and food security.
Keywords: LAMP; PCR; Ustilaginoidea virens; false smut; rice; spore trap.
Copyright © 2024 Arumugam Gopalakrishnan, Chellappan, Ayyanar, Ramasamy, Santhosh Ganapati and Nagaranai Karuppasamy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Azizi M., Motamedi H., Hossainpour H., Abiri R., Kashef M., Ahmadi K., et al. (2022). Rapid detection of vanA resistance gene from E. faecalis clinical isolates using duplex loop-mediated isothermal amplification and triplex PCR assay. Bio. Med. Res. Int. 2022:4384196. doi: 10.1155/2022/4384196, PMID: - DOI - PMC - PubMed
-
- Baite M. S., Raghu S., Prabhukarthikeyan S. R., Mukherjee A. K., Bag M. K., Jena M., et al. (2020). Yield loss assessment in rice (Oryza sativa) due to false smut infection. Indian J. Agric. Sci. 90, 361–364. doi: 10.56093/ijas.v90i2.99023 - DOI
-
- Boutry C., Bohr A., Buchleither S., Ludwig M., Oberhänsli T., Tamm L., et al. (2023). Monitoring spore dispersal and early infections of Diplocarpon coronariae causing apple blotch using spore traps and a new qPCR method. Phytopathology 113, 470–483. doi: 10.1094/PHYTO-05-22-0183-R, PMID: - DOI - PubMed
-
- Calderon C., Ward E., Freeman J., McCartney A. (2002). Detection of airborne fungal spores sampled by rotating-arm and Hirst-type spore traps using polymerase chain reaction assays. J. Aerosol Sci. 33, 283–296. doi: 10.1016/S0021-8502(01)00179-3 - DOI
-
- Carisse O., Levasseur A., Van der Heyden H. (2012). A new risk indicator for botrytis leaf blight of onion caused by Botrytis squamosa based on infection efficiency of airborne inoculum. Plant Pathol. 61, 1154–1164. doi: 10.1111/j.1365-3059.2012.02594.x - DOI
LinkOut - more resources
Full Text Sources

