Potential role of TWIST1 and its methylation in bladder urothelial carcinoma
- PMID: 39697731
- PMCID: PMC11651768
- DOI: 10.21037/tcr-24-1029
Potential role of TWIST1 and its methylation in bladder urothelial carcinoma
Abstract
Background: Bladder urothelial carcinoma (BLCA), like other cancers, is strongly associated with genetic and epigenetic changes. TWIST1 is an epithelial-mesenchymal transition (EMT) promoter that has been linked to the development of many malignancies. It is still unclear, however, what role TWIST1 plays in BLCA, and the relationship between TWIST1 transcript levels and its promoter methylation and immune infiltration has been reported even less. This study aimed to reveal the potential role of TWIST1 promoter methylation-related changes in BLCA.
Methods: Transcriptional expression data of TWIST1 in BLCA were acquired from The Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO), and University of Alabama at Birmingham (UALCAN) databases. TWIST1 methylation levels and prognosis were sourced from Gene Expression Profiling Interactive Analysis 2 (GEPIA2), LinkedOmics, cBio Cancer Genomics Portal (c-BioPortal), MethSurv, and DNA Methylation Information Visualization Database (DNMIVD) databases. The methylation status of the BLCA-associated TWIST1 in preoperative and postoperative urinary exfoliated cells was subsequently analyzed using methylation-specific real-time fluorescence polymerase chain reaction, with validation of accuracy through pyrophosphate sequencing. Finally, from the Gene Set Cancer Analysis (GSCA) and Tumor-Immune System Interaction Database (TISIDB) databases, we obtained the association between TWIST1 transcript expression and DNA methylation and cancer immune infiltration and immunolabelling.
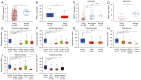
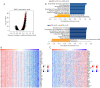
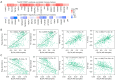
Results: Our study demonstrated that TWIST1 expression was down-regulated in BLCA, which was negatively correlated with DNA methylation. The association between TWIST1 promoter hypermethylation and the progression, staging, grading, and recurrence of BLCA is highly significant. Furthermore, we revealed that hypermethylation of both the preoperative and postoperative TWIST1 promoters is useful as a biomarker for monitoring BLCA recurrence, particularly when considering the methylation status of specific CpG sites. Additionally, we observed that TWIST1 expression, promoter methylation, and immune infiltration immunoreactive markers correlated significantly in BLCA.
Conclusions: We propose that TWIST1 holds great promise as a diagnostic and therapeutic target for BLCA, with the potential to influence tumor progression and patient prognosis through the regulation of immune cell infiltration. We hope these findings contribute valuable insights to the field of BLCA research.
Keywords: Bladder urothelial carcinoma (BLCA); TWIST1; epigenetics; methylation; prognosis.
2024 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1029/coif). The authors have no conflicts of interest to declare.
Figures




References
LinkOut - more resources
Full Text Sources
