Adjuvant therapy in renal cell carcinoma (RCC): progress, at last
- PMID: 39697753
- PMCID: PMC11651811
- DOI: 10.21037/tcr-23-2247
Adjuvant therapy in renal cell carcinoma (RCC): progress, at last
Abstract
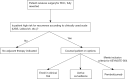
In the United States, there is expected to be about 82,000 cases of renal cell carcinoma (RCC) in 2024. At diagnosis, approximately 65% of patients with RCC will have disease localized to the kidney. For decades, the standard of care for patients with localized RCC has been surgery, which is often curative, followed by radiographic surveillance. However, after nephrectomy, patients may have up to 50% risk of recurrence. Thus, there has been a longstanding effort to reduce the recurrence of kidney cancer in the adjuvant setting after nephrectomy and/or metastasectomy. Over the past 30 years, a number of different therapeutic agents have been tested in the adjuvant setting including cytokines, autologous tumor cell vaccines, vascular endothelial growth factor (VEGF) pathway inhibitors, mammalian target of rapamycin (mTOR) inhibitors, and most recently immune checkpoint inhibitors (ICIs). The vast majority of these adjuvant trials in RCC have shown no significant clinical benefit for patients. In 2021, the KEYNOTE-564 trial demonstrated that adjuvant pembrolizumab improved progression-free survival and more recently showed an overall survival benefit for patients with high risk of recurrence of clear cell RCC (ccRCC). These findings have ushered in a new standard of care for patients with ccRCC at high risk of recurrence after nephrectomy. Here, we provide an overview of the major adjuvant trials in RCC, with a focus on ccRCC, and provide a framework for the management of patients with high risk localized ccRCC.
Keywords: Adjuvant therapy; KEYNOTE-564; clear cell renal cell carcinoma (ccRCC); immune checkpoint inhibition; tyrosine kinase inhibitors (TKIs).
2024 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2247/coif). M.N.S. reports receiving grants or contracts from Janssen Oncology, Advaxis, Bristol-Myers Squibb, Lilly, Seattle Genetics, Xencor, Tmunity, Exelixis, Bellicum Pharmaceuticals, Regeneron, Bicycle Therapeutics and AstraZeneca, all of which have provided payment to his institution; and also receiving consulting fees from Exelixis, Xencor, Janssen, Vaccitech, Merck, and Bristol-Myers Squibb/Medarex. K.R. reports receiving funding from the 2023 Robert A. Winn Diversity in Clinical Trials Career Development Award, and a payment for serving as a steering committee for a phase III trial for Janseen. The other author has no conflicts of interest to declare.
Figures
References
-
- Albiges L, Powles T, Staehler M, et al. Updated European Association of Urology Guidelines on Renal Cell Carcinoma: Immune Checkpoint Inhibition Is the New Backbone in First-line Treatment of Metastatic Clear-cell Renal Cell Carcinoma. Eur Urol 2019;76:151-6. 10.1016/j.eururo.2019.05.022 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous