ABALON regulates mitophagy and 5-FU sensitivity in colorectal cancer via PINK1-Parkin pathway
- PMID: 39697760
- PMCID: PMC11651798
- DOI: 10.21037/tcr-24-933
ABALON regulates mitophagy and 5-FU sensitivity in colorectal cancer via PINK1-Parkin pathway
Abstract
Background: Growing evidence demonstrated that long non-coding RNAs (lncRNAs) are closely related with chemoresistance in colorectal cancer (CRC). Mitophagy serves as an essential factor to maintain the quality of tumor cells. However, it is unclear whether lncRNAs are involved in mitophagy regulation in CRC. The aim of this study is to evaluate whether lncRNAs are involved in regulating mitophagy and chemoresistance in CRC.
Methods: In this study, gain/loss of function was used to analyze the biological function influenced by apoptotic BCL2L1-antisense long non-coding RNA (ABALON). Western blot and JC-1 probe were carried out for detecting mitophagy. Chemosensitivity of CRC cells to 5-fluorouracil (5-FU) was determined using cell counting kit-8 (CCK-8), flow cytometry, colony formation and trans well assays.
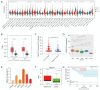
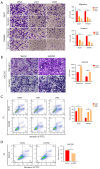
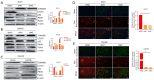
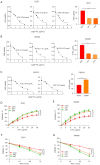
Results: We found that ABALON expression was increased in CRC, especially in consensus molecular subtype 1 (CMS1) and highly expressed ABALON was related with tumor differentiation, tumor node metastasis (TNM) staging, and lymph node metastasis (P<0.05). ABALON knockdown led to impaired proliferation and enhanced apoptosis in CRC. Mitophagy variations primed by ABALON enhanced mitochondrial homeostasis. The half maximal inhibitory concentration (IC50) of 5-FU in ABALON interference groups declined, while ABALON overexpression elevated IC50. Furthermore, defective mitophagy not only rescued the proliferation, metastasis, and apoptosis induced by ABALON overexpression, but also, enhanced the anti-tumor effect of 5-FU in vivo.
Conclusions: Collectively, our study proposed that ABALON potentiates CRC progression via PINK1/Parkin mediated mitophagy, and ABALON is a promising therapeutic target in reversing 5-FU resistance.
Keywords: 5-fluorouracil (5-FU); Colorectal cancer (CRC); long non-coding RNA (lncRNA); mitophagy; sensitivity.
2024 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-933/coif). The authors have no conflicts of interest to declare.
Figures



References
-
- André T, de Gramont A, Vernerey D, et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J Clin Oncol 2015;33:4176-87. 10.1200/JCO.2015.63.4238 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
