Efficacy of mycophenolate mofetil combined with topical 0.05% tacrolimus in high-risk keratoplasty: 1-year cohort study
- PMID: 39697894
- PMCID: PMC11589437
- DOI: 10.18240/ijo.2024.12.10
Efficacy of mycophenolate mofetil combined with topical 0.05% tacrolimus in high-risk keratoplasty: 1-year cohort study
Abstract
Aim: To investigate the efficacy of systemic mycophenolate mofetil (MMF) as an adjunct in combination with topical tacrolimus (FK506) and corticosteroid eyedrops for preventing corneal graft rejection after high-risk keratoplasty (HRK).
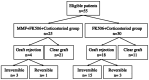
Methods: In this cohort study, 55 consecutive patients (55 eyes) from an eye center who met the criteria of HRK were included. The definition for HRK includes large grafts of no less than 9 mm diameter, vascularized cornea of two or more quadrants, regrafting, or eccentric grafts. After penetrating keratoplasty, 25 patients treated with systemic MMF in combination with 0.05% FK506 and tapering corticosteroid eyedrops were enrolled in Group 1 from October 2019. Thirty patients receiving postoperative treatment with 0.05% FK506 and tapering corticosteroid eyedrops alone were enrolled in Group 2 from January 2018 to September 2019. All participants were closely monitored after surgery, and episodes of graft rejection and relevant clinical data were collected and assessed over a one-year follow-up period.
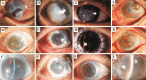
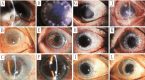
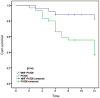
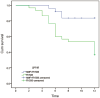
Results: After a follow-up of 9.6±3.2mo, graft rejection episodes occurred in 4 cases (16%) in Group 1 and 18 cases (60%) in Group 2. One reversible and 3 irreversible graft rejections occurred in Group 1, while 3 reversible and 15 irreversible rejections occurred in Group 2. Kaplan-Meier analysis revealed that 82.5% of grafts in Group 1 and 37.1% in Group 2 did not experience corneal graft rejection (P<0.01, log-rank test). The clear graft survival rate was 83.6% in Group 1 and 36.7% in Group 2 (P<0.01, log-rank test) within one year of follow-up. No severe systemic side effects were observed in either group during the follow-up period.
Conclusion: The triple treatment regimen consisting of MMF, topical FK506, and corticosteroid eyedrops represents a promising strategy for effectively preventing graft rejection and improving graft survival in patients with HRK.
Keywords: high-risk keratoplasty; immunosuppressants; mycophenolate mofetil.
International Journal of Ophthalmology Press.
Conflict of interest statement
Conflicts of Interest: Zhang JY, None; Yu K, None; Jiang XY, None; Liang JX, None; Zhou SY, None.
Figures
References
-
- Pramanik S, Musch DC, Sutphin JE, Farjo AA. Extended long-term outcomes of penetrating keratoplasty for keratoconus. Ophthalmology. 2006;113(9):1633–1638. - PubMed
-
- Muraine M, Sanchez C, Watt L, Retout A, Brasseur G. Long-term results of penetrating keratoplasty. A 10-year-plus retrospective study. Graefes Arch Clin Exp Ophthalmol. 2003;241(7):571–576. - PubMed
-
- Infantes Molina EJ, Celis Sánchez J, Tenias Burilllo JM, Diaz Valle D, Benítez-Del-Castillo JM, Mesa Varona D, Avendaño-Cantos E. Deep anterior lamellar keratoplasty versus penetrating keratoplasty in corneas showing a high or low graft rejection risk. Eur J Ophthalmol. 2019;29(3):295–303. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
