Hematological Changes in Platelet Apheresis Donors: An Observational Study
- PMID: 39697965
- PMCID: PMC11653975
- DOI: 10.7759/cureus.73907
Hematological Changes in Platelet Apheresis Donors: An Observational Study
Abstract
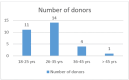
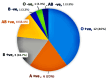
Background The demand for platelet concentrates is increasing for treating patients in clinics such as oncology. Single donor platelets (SDPs) collected through apheresis offer a lower risk of transfusion-related complications. Aim This observational study aims to evaluate hematological changes in donors before and after platelet donation via apheresis, shedding light on donor safety and eligibility criteria. Methods This was a pilot study involving 30 plateletpheresis procedures. Hematological parameters were measured before and after donation, including platelet count and hematocrit. Results All donors were male, with an age range of 18-45 years. Blood group distribution among donors was O+ve (12), A+ve (6), B+ve (5), AB+ve (4), B-ve (1), O-ve (1), and AB-ve (1). The mean fall in platelet count after donation was 50,833/mm3. The overall mean fall in hematocrit was 1.16%, with a range of 0.5-2%. For donors with a platelet yield of 3.0x1011, the average hematocrit reduction was 1.13%, whereas for donors with a yield of 3.5x1011, the reduction was 1.56%. Conclusion Automated cell separators have significantly improved SDP quality and collection efficiency. The correlation between platelet yield and pre-donation platelet count underlines the importance of personalizing collection parameters.
Keywords: donor; hematocrit; platelet; plateletpheresis; single donor platelets.
Copyright © 2024, Sharma et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Institutional Ethical Committee, BLDE (Deemed to be University), Shri B M Patil Medical College, Hospital & Research Centre issued approval BLDE(DU)/IEC/613/2022-23, dated August 26, 2022. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- A prospective quality evaluation of single donor platelets (SDP) - an experience of a tertiary healthcare center in India. Pandey P, Tiwari AK, Sharma J, Singh MB, Dixit S, Raina V. https://pubmed.ncbi.nlm.nih.gov/22365925/ Transfus Apher Sci. 2012;46:163–167. - PubMed
-
- Single donor versus pooled random donor platelet concentrates. Ness PM, Campbell-Lee SA. https://journals.lww.com/co-hematology/abstract/2001/11000/single_donor_.... Curr Opin Hematol. 2001;8:392–396. - PubMed
-
- Pooled platelet concentrates provide a small benefit over single-donor platelets for patients with platelet refractoriness of any etiology. Chu YH, Rose WN, Nawrot W, Raife TJ. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8142527/ J Int Med Res. 2021;49:3000605211016748. - PMC - PubMed
-
- Use of single donor platelets. Kirkley SA, Blumberg N. https://www.sciencedirect.com/science/article/abs/pii/0268960X9490074R?v.... Blood Rev. 1994;8:142–147. - PubMed
-
- Pooled platelet concentrates: maybe not fancy, but fiscally sound and effective. Sweeney JD, Petrucci J, Yankee R. https://www.sciencedirect.com/science/article/abs/pii/S0955388697000568?.... Transfus Sci. 1997;18:575–583. - PubMed
LinkOut - more resources
Full Text Sources